PROPERTIES OF GLUTAMATE RECEPTORS
The amino acid L-Glutamate
is a neurotransmitter in many central excitatory pathways. In
addition, certain other naturally-occuring amino acids, such as L-Aspartate and L-Homocysteate
also have excitatory actions. All of these exert their actions via a
number of receptors. The
classification and identification of these receptors has been the
subject of intense study by many
workers over several decades. An outline of this work is presented
below.
The excitatory amino acid receptors can be grouped into
ionotropic receptors (i.e. those where
receptor activation is directly coupled to a membrane ion channel) and
metabotropic receptors
(i.e. those where receptor activation is coupled to an intracellular
biochemical cascade: this may
eventually lead to opening or closing of membrane ion channels, amongst
other effects). The
ionotropic receptors were the first to be classified pharmacologically,
largely due to the efforts of
Watkins and his colleagues, and the broad scheme of NMDA receptors and non-NMDA
(AMPA/kainate) ionotropic receptors, based on responses evoked by the
selective agonists
NMDA, AMPA and kainate is still in use. Subsequently, metabolic
responses to excitatory amino
acid agonists were discovered and this ultimately led to the
characterisation of the metabotropic
glutamate receptors (mGluRs)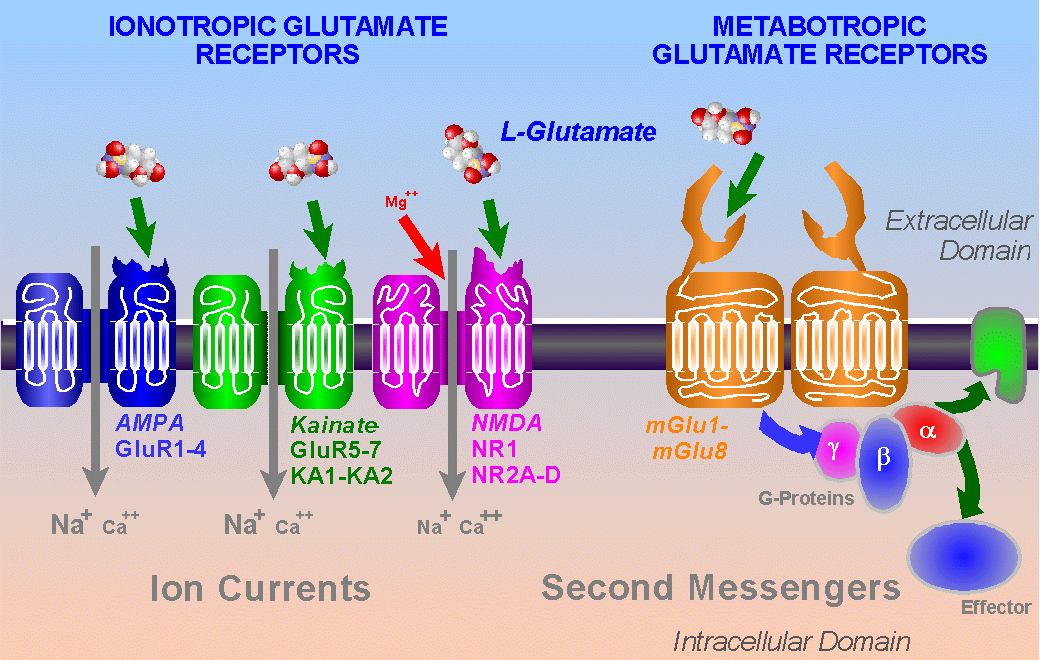
AMPA/Kainate receptors
These receptors were originally classified by their activation by the
agonists quisqualate and
kainate, but not NMDA. The use of quisqualate as an agonist for these
receptors has now been
abandoned in favour of the more selective agonist AMPA, and these
receptors are thus referred to
either as 'non-NMDA ionotropic receptors' or 'AMPA/kainate receptors'.
It is noteworthy that
kainate itself can activate AMPA receptors, and AMPA can activate most
of the kainate
receptors. The AMPA analogue ATPA has been shown to be a relatively selective agonist at GluR5-containing kainate receptors.
Molecular biological techniques have so far revealed the
existence of four glutamate
receptor subunits (GluR1-GluR4) which can be regarded as AMPA receptor
subunits, and five
receptor subunits which can be regarded as kainate receptor subunits
(GluR5-GluR7 and KA1,
KA2). Both of these subunit groups can form homomeric and heteromeric
channel assemblies
with other members of their groups. Furthermore, immunoprecipitation
experiments demonstrate
that GluR6 and/or GluR7 subunits assemble with KA2, but not with
GluR2-GluR4. All of the
AMPA and kainate receptors appear to be blocked by the competitive
AMPA/Kainate
antagonists CNQX and NBQX, although these antagonists do appear to show
selectivity to native
AMPA receptors and some novel non-competitive antagonists (e.g.
GYKI52466) also appear to
have selectivity for AMPA receptors compared to kainate receptors. The
dye Evans Blue has been
shown to block all combinations of GluR1-GluR4 apart from homomeric
GluR3 and GluR6. More recently, it has been shown that GluR6 but not
GluR2/4 can be antagonised by the novel
compound NS-102, and that LY328884 can antagonise kainate receptors that contain GluR5.
Apart from GluR2, the cloned AMPA receptors have a non-linear
voltage relationship and are
relatively Ca2+ permeable. However, in heteromeric AMPA receptors the
linear voltage
properties and Ca2+-impermeability of GluR2 are dominant. In most CNS
neurones
AMPA/kainate responses show little Ca2+ permeability and this is in
accordance with the
widespread expression of GluR2 throughout the CNS. The peculiar
property of the GluR2
subunit appears to be due to RNA editing at one site. Each of the AMPA
receptor subunits can
exist in two forms due to alternative splicing ('flip' and 'flop'
forms), the efficacy of L-glutamate
being higher at the 'flip' form.
GluR5-GluR7 are thought to correspond to the low-affinity
kainate receptors, whereas KA1 and
KA2 correspond to the so-called high-affinity kainate receptors.
Homomeric GluR7, KA1 or
KA2 receptors do not appear to give agonist responses, but this may
(for example) be due to a
very rapid desensitisation which might obscure responses. However,
heteromeric complexes of
KA2 with GluR5 or GluR6 do form functional receptors, and it is
noteworthy that KA2/GluR6
shows a substantial response to AMPA.
NMDA receptors
The NMDA-receptor-channel-complex
has been extensively studied, and it is known that they
have a relatively higher Ca2+ permeability than the non-NMDA ionotropic
receptors, they are
blocked by Mg2+ in a voltage- dependent manner, they have a requirement
for glycine (or similar
ligand) as a co-agonist, and they have modulatory sites for polyamines,
reducing agents, Zn2+ and
protons. These receptors can be antagonised in a competitive manner by
a growing number of
substituted five-carbon and seven-carbon chain glutamate analogues such
as D-2-amino-
phosphonopentanoic acid (AP5 or
APV) and 3-((+)-2- carboxypiperazin-4-yl)
-propyl-1-phosphonate (CPP), and in a non- competitive manner by
phencyclidine and the dissociative
anaesthetic ketamine.
Molecular biological techniques have revealed that the
NMDA-receptor-channel-complex
comprises two subunits (NR1 and NR2). There are eight splice variants
of NR1, and it is thought
that NR1 is a component of all native NMDA receptors, although NR1
subunits can be assembled
into homomeric NR1 channels. There are four NR2 subunit types
(NR2A-NR2D), which when
co-expressed with NR1 are thought to form native NMDA-receptor-channel
complexes. The
different NR2 subunits appear to confer different physiological and
pharmacological properties on
the receptors: for example, NR1-NR2C channels are more sensitive to
Mg2+ blockade and display
the highest affinity sites for glycine binding compared to other
heteromeric channels, whereas the
NR1-NR2A channel differs from the others in its response to reducing
agents. The NR1 subunit
is ubiquitous throughout the CNS, whereas there is a differential
distribution of NR2 subunits: for
example NR2C expression levels are high in the cerebellum, but low
elsewhere. NR2A and
NR2B are found in the thalamus, although NR2A is distributed more
prominently in the lateral
thalamic nuclei, especially the ventrobasal complex and NR2D is
expressed early during
development rather than in the adult. It is thought that the NR1-NR2A
complex in particular
displays a higher affinity for competitive NMDA antagonists than for
agonists, and NR1-NR2A
has the fastest offset decay time following pulsatile L-glutamate
application.
At the time of writing, there are known to be at least eight
metabotropic glutamate receptors
(mGluR1-8), which can be placed into three groups on the basis of
sequence homology, agonist
pharmacology, and coupling to intracellular transduction mechanisms.
Group I comprises
mGluR1 and mGluR5 (In addition, there are splice variants of mGluR1 and
mGluR5), and these
receptors appear to be coupled to postsynaptic inositol phosphate
metabolism. Group II
comprises mGluR2 and mGluR3, and Group III comprises mGluR4, mGluR6,
mGluR7 and
mGluR8. Groups II and III can couple to an inhibitory cAMP cascade in
many expression
systems, but may also couple to other transduction mechanisms under
physiological conditions. The Group II and III receptors have been
suggested to mediate presynaptic actions of glutamate
in several brain areas, although this does not preclude the possibility
that these receptors may also
mediate postsynaptic effects in some locations. Similarly, there is
evidence that Group I receptors
may be found in what appear to be presynaptic locations in some cases.
Within the thalamus of
adults, in situ hybridisation studies have shown
particularly prominent expression of mGluR1 ,
mGluR4 and mGluR7. mGluR3 mRNA is highly expressed in neurones of the
thalamic reticular
nucleus.
Historically, use of the agonist (1S,3R)-ACPD,
which acts at most of the known metabotropic receptors with
a varying degree of potency, has made it very difficult to draw
conclusions about the physiological
role(s) of the various metabotropic receptors when agonists are applied
to complex neural
systems which almost certainly contain a variety of receptors at
different pre- and postsynaptic
loci. These difficulties were exacerbated by the lack of selective
competitive antagonists to
the metabotropic receptors. Several compounds (e.g.
L-AP3, L-AP4, L-aspartic acid-ß-hydroxamate) had been
suggested as antagonists on the basis of neurochemical studies, but
electrophysiological experiments with these compounds indicate that
they cannot be regarded as
antagonists and are in some cases full agonists (e.g. L-AP4). Thus, functional studies
where these
compounds have been used must be considered with caution. The situation has however improved as more selective agonists (e.g. 3,5-dihydroxyphenylglycine, CCG-I, LY354740, DCPG) and
antagonists (e.g. 4-CPG, MCPG, MPPG, LY367385, MPEP, LY341495) have become
available for use in studies
of synaptic function.
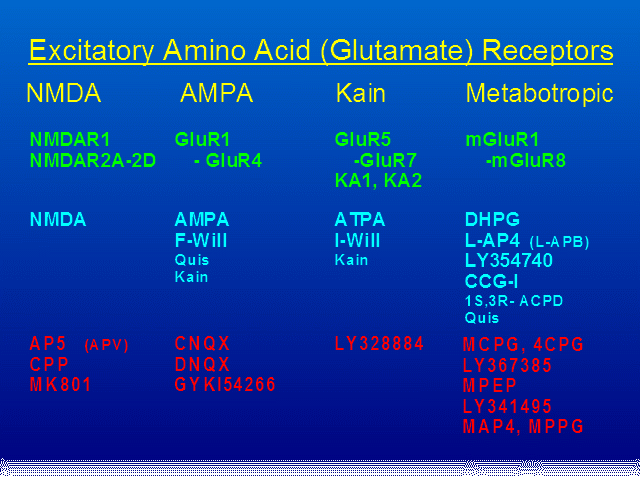
Green: Cloned Receptors. Cyan: Agonists.
Red: Antagonists.


