Highlighting important considerations during the "Therapeutic Design & Development" stage of the Small Molecules translational pathway.
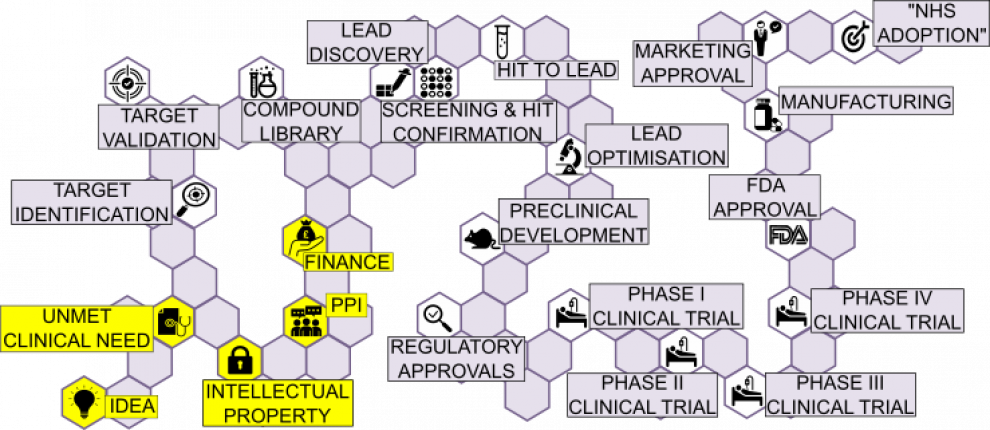
 IDEA:
IDEA:
Target Product Profile (TPP) – captures the ‘benefit’ of the proposed therapeutic and key considerations in the strategic development of the product (incl. technical, scientific and medical information required to satisfy key stakeholders (regulators and funders)).
It's common for a team to not know the answers to all questions at the start of the project, however this will be refined and developed as the project progresses.
UCL Support:
The UCL Translational Research Office Drug Discovery Group (TRO DDG) have extensive knowledge and experience of the Small molecules landscape, and can provide advice on early considerations such as intended use and drug description etc, to guide development, as well as longer-term considerations for adoption i.e. help you produce and develop your TPP.
 UNMET CLINICAL NEED:
UNMET CLINICAL NEED:
As part of the TPP, comprehensively research and define the potential for benefit of the proposed therapeutic over current ‘standard of care’.
UCL Support:
Small Molecule Therapeutic Innovation Network is a network of those focused on small molecule drug discovery at UCL. UCL and partner NHS Trusts are globally leading innovators in the discovery, design, development, screening, clinical delivery of small molecule drugs.
The Small Molecule TIN committee expertise in small molecule research which spans multiple therapeutic areas, working across disciplinary boundaries and with our NHS partners to bring novel candidates to the market, and breathe new life into existing candidates.
 INTELLECTUAL PROPERTY:
INTELLECTUAL PROPERTY:
Engage with Business experts to ascertain if you have a novel invention, ensure freedom to operate and to protect foreground/arising intellectual property.
UCL Support:
If you believe you have an innovative, patentable technology, it’s important you contact UCLB before any form of public domain disclosure.
UCLB are experts that provide confidential support for researchers at UCL, to understand the value of your invention and the best approach to protecting your intellectual property (IP), advising on strategies to commercialise your research such as through forming a spin out company or licensing to a third party.
All researchers with potentially commercialisable research results should fill out a confidential Invention Disclosure Form (IDF) and submit it to their UCLB Business Manager: http://www.uclb.com/for-researchers/do-you-believe-you-have-a-novel-invention/
 PATIENT & PUBLIC INVOLVEMENT (PPI):
PATIENT & PUBLIC INVOLVEMENT (PPI):
Understanding the patient and clinician needs - Does your TPP meet all of the essential requirements expressed by frontline clinical care providers, patient and public contributors (PPI)?
Important PPI actions:
- Research is carried out ‘with’ members of the public – Advisory members of a project steering group
- Early PPI engagement will help raise awareness of the therapeutic during development and potentially facilitate patient recruitment for subsequent clinical evaluation, possibly even financial investment
- Timely identification of future needs or opportuniti
Further information on PPI can be found via INVOLVE: www.invo.org.uk/
UCL Support:
The UCL Translational Research Office (TRO) can help with the timely identification of future needs or opportunities for diversifying, as well as advising on appropriate patient groups and charities to consider.
 FINANCE:
FINANCE:
The development of small molecule compound to clinical level has significant costs that must be met. Additionally, VAT needs to be added for manufacturing and/or services not resulting in a medicinal product.
UCL Support:
The UCL Translational Research Office supports UCL researchers in attracting and managing public, commercial, investor and philanthropic funding for the development of small molecules and pre-clinical studies. These projects are then either spun out into a company or partnered with a pharmaceutical company, to help progress the project to clinical studies.
Contact the UCL Translational Research Office (TRO)
Clinical studies need to be appropriately costed in partnership with the Joint Research Office (JRO) or appropriate CTU.
 Close
Close

