Final candidate molecules must possess a well-defined set of properties before they are considered suitable for testing in humans.
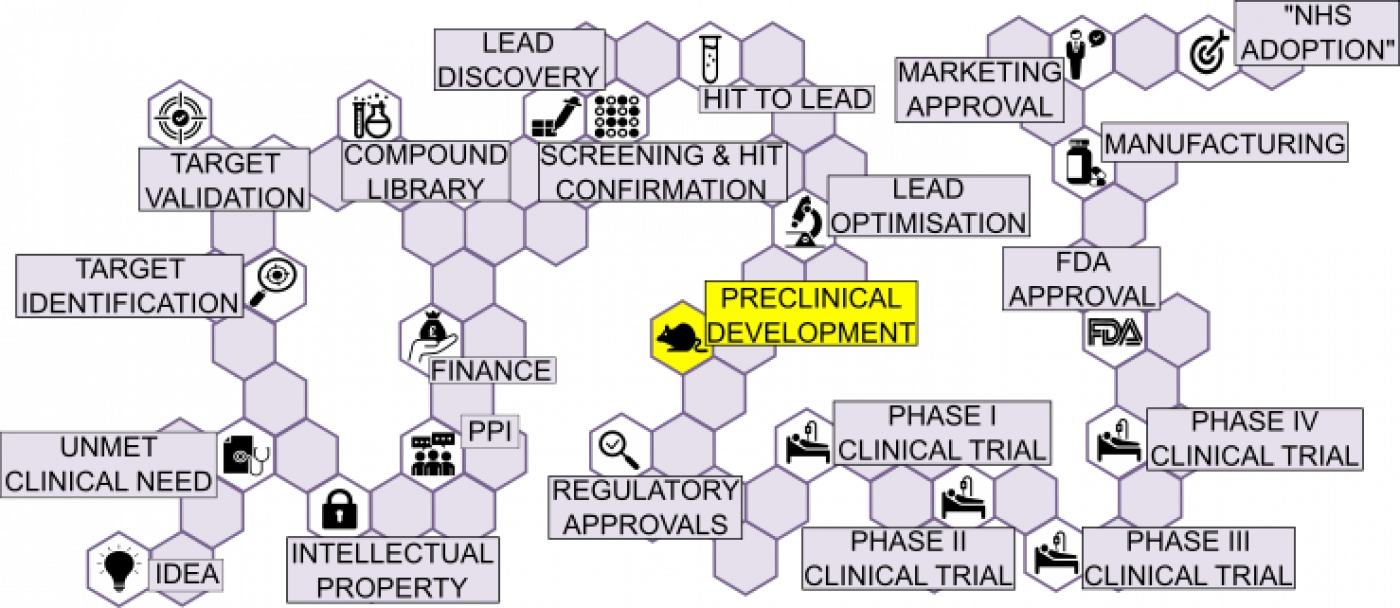
 PRECLINICAL DEVELOPMENT:
PRECLINICAL DEVELOPMENT:
Millions of compounds may have been tested in the original screen, followed by as many as 500 compounds synthesised in the subsequent optimisation, leading to only 10-20 compounds being tested in advanced models of disease and finally, only 1-2 compounds selected for testing in humans.
Those final candidate molecules need to meet certain properties before considered as suitable for testing in humans.
- Chemical properties: the compound should be stable and synthesis of the compound is straight forward so that it is easily scalable.
- Physicochemical properties: the compounds should be soluble and preferably meet the Lipinski ‘Rule of 5’.
- Pharmacological properties: the compound should show selectivity towards the target and bind to the target, in both the in vitro experiments and the in vivo experiments (animal models).
- Pharmacokinetic properties: the compounds should be bioavailable, display appropriate half-life and the correct distribution in animals. The mode of action of the compound should be well known.
- Safety and toxicity potential: the compound should not show toxicity, such as cardiac toxicity, genotoxicity, and hepatotoxicity, in both in vitro or in vivo experiments.
When a compound passes all the necessary procedures, this compound undergoes Pharmaceutical formulation, which is the process where an active compound is combined with different chemical and/ or biochemical substances to produce the final medicinal product.
 Close
Close

