SIG mainly carries out surgeon-led trials, with an emphasis on breast cancer, urology, and hepato-pancreato-biliary disease. We specialise in pragmatic phase III trials, which are practice changing.
We are involved in the following trials.
ADEPTS
Accelerated Diagnosis of neuro Endocrine and Pancreatic Tumours is an early biomarker study which aims to detect pancreatic cancer at a much earlier stage.
- More about ADEPTS

Pancreatic cancer is difficult to diagnose as many people have vague symptoms or no symptoms at all. It is mostly diagnosed at a late stage when it has already spread and can lead to poor outcomes for patients. Just 1% of those diagnosed will live for ten years or more after diagnosis.
Currently the best non-invasive tests for pancreatic cancer are MRI and CT scans, but they cannot accurately detect early cancer or reliably tell the difference between cancer and pre-cancerous growths. By developing a urine and blood test, we hope to detect pancreatic cancer at an earlier stage to allow successful treatment.
Previous studies have identified biomarker molecules in blood and urine. This study aims to develop a prospective biobank and an early diagnostic tool that can differentiate early PDAC (pancreatic ductal adenocarcinoma), PNETs (pancreatic neuroendocrine tumours) and high-risk pancreatic lesions from benign disease, by combining a risk factor / early symptom electronic clinical decision support tool (e-CDST) with novel panels of blood and urine biomarkers of early disease. This diagnostic tool may then be used for surveillance of high-risk populations and triage of patients with non-specific symptoms concerning for pancreatic cancer.
The ADEPTS study (formally TRANSBIL: Translational research in biliary tract and pancreatic diseases) will recruit 2,500 subjects.
- Sponsor: University College London
- Funder: Pancreatic Cancer UK
Publications
Pereira, S., Hippisley-Cox, J., Hsuan, J., Williams, N. et al (2020). ADEPTS (Accelerated Diagnosis of neuroEndocrine and Pancreatic TumourS) and EDRA (Early Diagnosis Research Alliance). Pancreatology, Volume 20, Issue 8, 2020, Page e14.
CADMUS
Multi-parametric ultrasound imaging to detect and rule-out clinically significant prostate cancer.
- More about CADMUS

We will look at men who have already had one prostate biopsy and are referred for risk stratification.
At present, MRI scanning is used to identify areas of the prostate which may contain cancer and would warrant further investigation with biopsy. MRI is a relatively expensive test and is not suitable for all men such as those with metal implants, poor kidney function and claustrophobia.
If this could use ultrasound scanning, which is perhaps half the cost of MRI, the economic gains might be considerable. Ultrasound could also be performed in the outpatient setting by the urologist, removing a visit to hospital.
The study will be conducted at University College Hospital, and six other sites. All participants will undergo a multi-parametric MRI scan (the normal diagnostic pathway) as well as a multi-parametric ultrasound scan in two outpatient visits. Any abnormalities on these two scans considered suspicious for cancer will be biopsied under local anaesthetic and optional sedation. The biopsy results for abnormalities detected by ultrasound will be compared with the biopsy results for those abnormalities seen on MRI to answer our primary research question.
There are seven UK sites, and this trial will register 400 patients, of which 275 will be randomised.
- Sponsor: University College London
- Funder: Prostate Cancer UK and the Moulton Foundation
- ClinicalTrials.gov Identifier: NCT02712684
Publications
Grey A, Scott R, Charman S, Emberton M, et al. (2018). The CADMUS trial ‐ Multi‐parametric ultrasound targeted biopsies compared to multi‐parametric MRI targeted biopsies in the diagnosis of clinically significant prostate cancer. Contemp Clin Trials.
Grey ADR, Scott R, Shah B, Brew-Graves C, Emberton M, et al. (2022). Multiparametric ultrasound versus multiparametric MRI to diagnose prostate cancer (CADMUS): a prospective, multicentre, paired-cohort, confirmatory study. The Lancet Oncology, 23.3, 428-438.
DETECT I
A prospective observational study to determine the negative predictive value of UroMark to rule out the presence of bladder cancer in patients with haematuria.
- More about DETECT I
DETECT I is a multicentre observational study to assess whether the test called UroMark can detect bladder cancer in patients who are being investigated for haematuria (blood in the urine). The test detects changes in the DNA of cells which are present in urine. Changes in DNA, called mutations or epigenetic alterations, are present in cells collected from a urine sample if cancer is present.
The study will recruit men and women who have been referred to hospital to have tests because of the finding of either visible or non-visible blood in the urine.
Everyone who agrees to take part will be receiving the normal investigations for haematuria in outpatient clinics. Subjects will be asked to provide three urine samples and complete a brief questionnaire.
- Sponsor: University College London
- Funder: Medical Research Council
- ClinicalTrials.gov Identifier: NCT02676180
Publications
Tan, W.S., Feber, A., Dong, L., Brew-Graves, C., Kelly, J.D. et al. (2017). DETECT I & DETECT II: a study protocol for a prospective multicentre observational study to validate the UroMark assay for the detection of bladder cancer from urinary cells. BMC CANCER, 17, ARTN 767.
DETECT II
A multicentre observational study to determine the sensitivity of the UroMark assay, a urine test, to detect new and recurrent low, intermediate and high-grade bladder cancer.
- More about DETECT II
DETECT II will recruit consecutive patients attending haematuria clinics as well as patients referred to the check cystoscopy clinics. They will provide a urine sample when they join and be asked to provide samples from home every 3 months prior to each surveillance cystoscopy for 24 months.
We aim to recruit 400 patients. The standard of care assessments will be performed as usual for this disease group and the results will be used to confirm whether it is bladder cancer and their stage and grade.
Patients will be followed-up for 24 months. They will also be asked to complete a simple questionnaire. Except for the urine sample that will be provided by patients from home and the questionnaire, all treatment and assessments are according to standard care.
- Sponsor: University College London
- Funder: Medical Research Council
- ClinicalTrials.gov Identifier: NCT02781428
Publications
Tan, W.S., Feber, A., Dong, L., Brew-Graves, C., Kelly, J.D. et al. (2017). DETECT I & DETECT II: a study protocol for a prospective multicentre observational study to validate the UroMark assay for the detection of bladder cancer from urinary cells. BMC CANCER, 17, ARTN 767.
ENSEMBLE
Enabling the study of metabolism in breast cancer through collection of fresh-tissue biopsies.
- More about ENSEMBLE
Often, cancer cells will use different nutrients to healthy cells to grow, or they will use the same nutrients but in an altered way. The behaviour of individual cancers seems to be influenced by how they process these nutrients. Previous studies have already shown that if breast cancer cells cannot access specific nutrients, they cannot grow. Understanding more about this in individual tumours may allow us to identify new treatment targets and improve breast cancer treatment in the future. This is what ENSEMBLE hopes to address.
Enrolled patients will receive up to two additional ‘research biopsies’, depending on their treatment plan. Patients who are due to receive treatment before their surgery (neo-adjuvant treatment) will receive two research biopsies – before their neo-adjuvant treatment starts and at the time of surgery (intra-operatively). Patients not having neo-adjuvant treatment will receive one research biopsy at the time of surgery.
The research biopsy procedure is the same as that which forms the standard of care (to inform a cancer diagnosis). It is termed a 'research biopsy' as it is an additional procedure purely for research purposes. Each biopsy will collect a maximum of four 'cores' (tissue samples) of breast tumour tissue. This will be analysed to look for patterns in the cancer cell metabolism.
Some analysis for this study will involve the use of mice as a model for human cancer, which have been specifically bred in a laboratory for research purposes. All work with mice will comply with the legislation surrounding the use of laboratory animals in research.
The primary outcome (objective) is the successful collection of biopsies. The secondary outcome is understanding how specific 'events' that work in favour of breast cancer cells alter their metabolism, allowing them to grow uncontrollably. This allows the team to identify areas of weakness in cancer cell metabolism, with a view to identifying new targets for cancer therapies.
The study will be carried out at six hospitals in the UK:
- University College London Hospitals NHS Foundation Trust (UCLH)
- Royal Free London NHS Foundation Trust
- Whittington Health NHS Trust
- Barts Health NHS Foundation Trust
- Guy’s and St Thomas’s NHS Foundation Trust
- King’s College Hospital NHS Foundation Trust
The study aims to enrol 240 patients across these sites. Once the patients have had their research biopsy/ biopsies, they will return to the standard of care, and are not required to complete any further activities specifically for the study. They will then be followed up remotely via NHS digital (national health registries) for clinical outcome data and to clarify information about medical history.
- Sponsor: University College London
- Funder: Cancer Research UK (CRUK) & Wellcome Trust
- Clinicaltrial.gov identifier: NCT03880097
Focal Recurrent Assessment and Salvage Treatment (FORECAST)
Investigating if new imaging tests can better identify cancer that has spread and identify areas of cancer inside the prostate.
- More about FORECAST
Radiotherapy is a common form of prostate cancer treatment in the UK. About 9,000 men in the UK undergo radiotherapy every year. One in four of these will experience failure of their treatment.
These men are usually offered hormone treatment, which can have side-effects. Few men are offered a further curative treatment to the prostate. This is due to lack of good imaging tests that can accurately detect whether cancer has returned inside the prostate or whether it has spread. Also, because radiotherapy damages the tissue surrounding the prostate tissue, healing following further treatment to the whole prostate can have side-effects.
Treating only the cancerous area in the prostate - focal therapy - may limit this damage with fewer side-effects.
We want to see if new imaging tests can better identify cancer that has spread and identify areas of cancer inside the prostate. Our new tests are whole-body MRI (for distant disease) and MRI-targeted biopsies (MRI-TB) (for local disease).
- First, we will compare the results of whole-body MRI to existing imaging tests (bone-scan, choline PET/CT) that try to rule-out distant spread.
- Second, we will compare the MRI-TB to a very detailed and accurate biopsy of the prostate called template prostate mapping which will show us where and how aggressive the cancer is.
- Third, if the cancer is confined to the prostate, we will treat men using focal salvage therapies using either heat therapy or freezing.
We believe these new imaging tests could better identify those who may benefit from early hormone treatment and those who will benefit from local salvage treatment. Our study may help justify carrying out a larger trial looking at how good focal salvage treatment is in controlling cancer in the medium and long-term.
- Sponsor: University College London (UCL)
- Clinicaltrial.gov: NCT01883128
Publications
Shah, T.T., Kanthabalan, A., Pavlou, M., Brew-Graves, C., et al. (2021). MRI and targeted biopsies compared to transperineal mapping biopsies for targeted ablation in recurrent prostate cancer after radiotherapy: Primary outcomes of the FORECAST trial.
Kanthabalan, A., Shah, T., Arya, M., Punwani, S., et al. (2015). The FORECAST study - Focal recurrent assessment and salvage treatment for radiorecurrent prostate cancer. Contemporary Clinical Trials, 44, 175-186.Leclerc, Q.J., Fuller, N.M., Keogh, R.H., Diaz-Ordaz, K., et al. (2021). Importance of patient bed pathways and length of stay differences in predicting COVID-19 hospital bed occupancy in England. BMC Health Services Research, 21, 566.
Shah, T. (2021). The role of minimally invasive ablative therapies in the treatment of primary and radio-recurrent prostate cancer. (Doctoral dissertation), UCL (University College London).
Shah, T., Kanthabalan, A., Otieno, M., Pavlou, M., Omar, R., et al. (2022). Magnetic Resonance Imaging and Targeted Biopsies Compared to Transperineal Mapping Biopsies Before Focal Ablation in Localised and Metastatic Recurrent Prostate Cancer After Radiotherapy. European Urology.
Focal Therapy for Prostate Cancer using HIFU (INDEX)
A multi-centre prospective single arm intervention trial evaluating focal therapy using high intensity focused ultrasound (Sonablate 500) for localised prostate cancer. Can we successfully treat men with localised prostate cancer by reliably destroying the cancer areas that are clinically important using high intensity focused ultrasound (HIFU)?
- More about INDEX
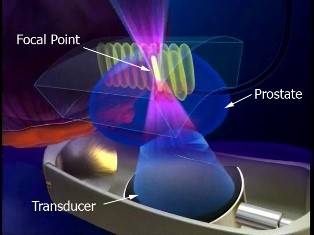
In most men, the prostate cancer has not spread outside of the prostate to other parts of the body. When this is the case, most patients are offered two main options:
- A 'wait and see' policy which involves regular blood tests and re-sampling of prostate tissue (biopsies) to check whether the cancer is progressing and requires more 'active' treatment.
- Surgery or radiotherapy to treat the whole prostate.
These treatments usually eliminate the cancer but can also cause side-effects such as urinary leaking, impotence, and bowel trouble. A potential option is to treat only the part of the prostate that contains the cancer, known as focal therapy. Results from earlier smaller trials have been promising, with most men free of cancer and major side-effects one year after focal therapy treatment. We need to explore this idea in a larger group of men to confirm that this method can treat cancer in a safe and tolerable way.
The INDEX trial aims to treat 354 men within several centres that already use the type of treatment we are planning. HIFU (high intensity focused ultrasound) uses ultrasound waves to heat and kill small areas of tissue without damaging surrounding normal tissue. We will follow men up for two years after treatment within the trial, and over the long-term once the trial has finished (for up to 10 years). Men will receive periodic clinic visits with blood tests and questionnaires, then an MRI and biopsy of the treated area one year after treatment.
Can we successfully treat men with localised prostate cancer by reliably destroying the cancer areas that are clinically important, whilst not treating those areas that have no cancer or clinically unimportant areas of cancer, using high intensity focused ultrasound (HIFU)?
- Sponsor: University College London.
- Funder: Sonacare Medical and University College London Hospital.
- ClinicalTrials.gov Identifier: NCT01194648
Publications
Peek, M.C.L., Ahmed, M., Scudder, J., Baker, R., et al. (2018). High-intensity focused ultrasound in the treatment of breast fibroadenomata (HIFU-F trial). International Journal of Hyperthermia, 34 (7), 1002-1009.
Peek, M., Ahmed, M., & Douek, M. (2015). P088. Circumferential High Intensity Focused Ultrasound (HIFU) in the treatment of breast fibroadenomata: The HIFU-F trial. European Journal of Surgical Oncology (EJSO), 41 (6), S51.
Peek, M.C.L., Ahmed, M., Scudder, J., et al. (2016). High intensity focused ultrasound in the treatment of breast fibroadenomata: results of the HIFU-F trial. International Journal of Hyperthermia, 32 (8), 881-888.
Peek, M.C.L., Ahmed, M., Napoli, A., et al. (2015). Systematic review of high-intensity focused ultrasound ablation in the treatment of breast cancer. British Journal of Surgery, 102 (8), 873-882.
Ahmed, H.U., Sahu, M., Govindaraju, S.K., Emberton, M., et al. (2009). High Intensity Focused Ultrasound (HIFU) Hemiablation Trial in localised unilateral prostate cancer: interim results. European Urulogy Supplements, 8 (4), 334.
iROC
A phase III multicentre randomised controlled trial to compare the efficacy of Robotically Assisted Radical Cystectomy (RARC) and intracorporeal urinary diversion with Open Radical Cystectomy (ORC) in patients with bladder cancer.
- More about iROC
The study will recruit patients with non-muscle invasive bladder cancer (NMIBC) or muscle invasive bladder cancer (MIBC) who have selected radical cystectomy (RC) for treatment. The time of interest for measurement of the primary outcomes will be 90 days post-surgery. Eligible patients will include those receiving neo-adjuvant chemotherapy (typically gemcitabine and cisplatin) and those having either an ileal conduit or a neo-bladder reconstruction.
Patients who have selected RC after appropriate counselling and following a specialist multi-disciplinary team (SMDT) recommendation will be approached for consent for this study.
Consenting participants will be randomised 1:1 to either iRARC or ORC. Patients will be followed for a minimum of 90 days post-surgery.
Trial assessments will be conducted at baseline (before RC), while participants are on admission, and then five weeks, 12 weeks, 24 weeks, and one year post-surgery.
- Sponsor: University College London
- Supported by: The Urology Foundation, The Champniss Foundation
- ClinicalTrials.gov Identifier: NCT03049410
- ISRCTN: 13680280
Publications
Khetrapal, P., Catto, J., Ambler, G., Ricciardi, F., Kelly, J., et al. (2022). Results of the Intracorporeal Robotic vs Open Cystectomy (IROC) multi-centre randomised trial.
Catto, J.W.F., Kelly, J.D., Khetrapal, P., Ambler, G., Ricciardi, F., at al. (2022). Robot assisted radical cystectomy with intracorporeal urinary diversion versus open radical cystectomy: Results from the iROC prospective randomised controlled trial.
Khetrapal, P., Williams, N.R., Ambler, G., Sarpong, R., Kelly, J.D. (2019). The iROC trial: An RCT comparing intracorporeal robot-assisted vs open radical cystectomy for bladder cancer.
Catto, J.W.F., Khetrapal, P., Ambler, G., Sarpong, R., Khan, M. S., et al. (2018). Robot-assisted radical cystectomy with intracorporeal urinary diversion versus open radical cystectomy (iROC): protocol for a randomised controlled trial with internal feasibility study. BMJ Open, 8 (8), ARTN e020500.
Little Journey
A multi-centre randomised controlled trial assessing the effectiveness of the Little Journey app at reducing peri-operative anxiety compared to standard care. We explore if using our VR app at home before coming to hospital for surgery reduces anxiety in 3–12-year-old children and whilst asleep.
- More about Little Journey

Over half a million children have a general anaesthetic (go to sleep) for an operation in the UK each year. Around three-quarters become distressed or anxious before they go to sleep, which is linked to delays in recovery from surgery and later problems such as difficulty sleeping, nightmares and bedwetting.
Medicines and other treatments to calm children may either cause other problems or are very expensive. As a potential remedy, our team has developed Little Journey, a virtual-reality app that prepares children for going to hospital and their operation.
Our smartphone app comes with a disposable cardboard headset, which we provide free. It enables children to explore their hospital environment and meet animated doctors and nurses that move around in a natural way. The app shows children what the inside of the hospital looks like, the people they will meet, and the equipment they will see.
The trial will capture whether using our VR app at home before coming to hospital for surgery reduces anxiety in 3 to 12-year-old children and whilst asleep.
304 children will take part in the research. Half will be given our headset and VR app to use at home at least two-weeks before their surgery. The other half will be given only the headset which they can use for fun with other openly available VR apps.
We will measure how anxious children and parents are before the operation and up to four weeks after surgery. By comparing the level of anxiety between children who do and do not have access to the app, we will infer whether it is successful.
- Sponsor: University College London
- Funder: NIHR Research for Patient Benefit
Lymphatic mapping of Oropharyngeal Cancer (LOOC)
Getting accurate assessments on whether cancer has spread to the other side of the neck will give doctors better information on which side of the neck to treat.
- More about LOOC
Cancer at the back of the mouth and throat is usually only discovered after it has spread to lymph glands in the neck. This means that both the original tumour and the cancer that has spread must be treated. Doctors need to decide whether to treat one or both sides of the neck, as the cancer may have spread to both or may be likely to spread to another side in the future. They base this decision on things such as how aggressive the cancer is, whether the patient smokes and whether the HPV virus is present.
This difficult decision can mean some patients get treatment on both sides of the neck that they may not need, which can result in lasting problems with swallowing. Others receive treatment on one side only to have the cancer return on the other. LOOC hopes to give doctors more accurate information on whether to treat one side or both.
It will use a procedure called Sentinel Node Biopsy (SNB) to see whether the cancer has spread to the other side of the neck. The first part is imaging. A radioactive substance called 'Lymphoseek' is injected into the tumour. This moves into the lymph nodes, and those to which it moves first are the sentinel nodes. A handheld detector called a gamma camera can detect where these are. As part of standard care, patients are examined under anaesthesia (EUA) and when this happens, patients will have 'Lymphoseek' injected into these sentinel nodes. To make sure the gamma camera has identified the correct nodes, the patients will have a CT scan the next day.
Part two is surgery. This will involve a new group of patients who will have the same imaging procedure. Those with sentinel nodes in the other side of the neck will have them removed during the EUA and these nodes will be examined for signs of cancer. Some patients in this part with easily accessible tumours will have their tumour injected with ‘Lymphoseek’ under local anaesthetic a few days after their original injection. If this is acceptable to patients, it could mean that we could reduce the number or visits and general anaesthetics that they receive.
If this study is successful, we will have found a way to give doctors much better, more accurate information on what treatment to give and accelerate the treatment process for patients.
- Sponsor: University College London
- Funder: National Institute of Health and Research
- Clintrial.gov identifier: To follow
MIAMI: Safe Surgery for Multiple Breast Cancers
Can patients with multiple breast cancers in the same breast avoid mastectomy by having multiple lumpectomies to achieve equivalent rates of local breast cancer recurrence? A randomised controlled feasibility study.
- More about MIAMI

Current breast imaging methods mean that multiple breast cancers are being diagnosed in many women who are usually offered mastectomy to remove their whole breast with immediate or delayed breast reconstruction. These multiple smaller cancers may be treated using breast-conserving surgery, which currently occurs in about a quarter of women. Called a therapeutic mammoplasty, this surgery can remove each cancer and to remodel the breast tissue. It leaves scars similar to those seen after a standard breast reduction.
The MIAMI Trial investigates if breast-saving surgery is as safe as mastectomy in terms of controlling the rate of cancer returning in the same breast or armpit, or elsewhere in the body.
Currently, surgeons are unsure about the quality of the studies about the long-term safety of breast-saving surgery. Some studies suggest that breast-saving surgery may be as safe as mastectomy, but there may be a slightly increased 5 and 10-year risk (around 2%) of the cancer returning in the remaining breast tissue.
The potential safety of breast-saving surgery also depends on additional treatments of the breast tissue using radiotherapy and chemotherapy and/or endocrine treatments as well as bone strengthening drugs. All these treatments can work together to kill possible microscopic cancer cells in the breast and reduce the chances of any cancer recurring in the breast.
The study will also record women's quality of life, satisfaction with the appearance of their breasts, and the costs of the surgery types.
- Sponsor: University College London
- Funder: NIHR Research for Patient Benefit
- ClinicalTrials.gov: clinicaltrials.gov/ct2/show/NCT03514654
- ISRCTN: http://www.isrctn.com/ISRCTN17987569
Publications
Winters, Z.E., Horsnell, J., Schmid, P., Jones, J.L., Shaaban, A., et al. (2016). Time for a randomised clinical trial evaluating breast conserving surgery compared to mastectomy in ipsilateral mutlifocal breast cancer (MFBC). BREAST, 26 149-150.
OSTaRa
Gathering new EQ5D data (assessing quality and quantity of life) for women with early breast cancer, who have been treated with breast conserving surgery and radiotherapy.
- More about OSTaRa
For the National Institute of Health and Care Excellence (NICE) to recommend new treatments for use in the National Health Service, it needs information on whether the treatment works and whether it is good value for money.
Clinical trials reveal if the treatment works but determining value for money requires an economic analysis. These involve looking at costs (money and risks) and benefits (improved quality and quantity of life). NICE recommends a simple, standard questionnaire called the EQ5D to calculate the combination of quality and quantity of life. For women with early breast cancer, who have been treated with breast conserving surgery and radiotherapy there is no up to date EQ5D data in published journals for the use of developing guidelines for NICE and other reviews.
OSTaRa will approach at least 200 women in a 12-month period to collect completed EQ5D questionnaires. It will calculate the utility scores (combining quality and quantity of life) for this group of women in the United Kingdom and make this data publicly available for economists and other researchers to use.
OSTaRa comprises part of a research degree leading to a PhD qualification.
Pitstop
A randomised trial of treatment with pentoxifylline and tocopherol alongside best standard therapy (rehabilitation exercises) in individuals with radiotherapy-induced fibrosis of the head and neck.
- More about Pitstop
Most head and neck cancer are treated with a combination of surgery, radiotherapy, and chemotherapy. Fibrosis, the hardening of tissue, is a common irreversible side effect of radiotherapy. It is estimated that up to 60% of these individuals will experience persistent fibrosis as toxic effect of the radiotherapy including trismus (locked jaw) and dysphagia (difficulty swallowing).
These side effects have a big impact on one’s quality of life, due to impaired talking, eating, chewing and swallowing.
Pitstop is a feasibility 36-month randomised trial of treatment with pentoxifylline and tocopherol alongside best standard therapy (rehabilitation exercises) compared to best standard therapy alone in 50 individuals with radiotherapy-induced fibrosis of the head and neck. It is a two-centre study with individual randomisation 1:1 into the two arms.
The outcome of this trial is to find out whether subjects are willing to be randomised. Additional outcomes (secondary) will look at patient-centred measures, such as quality of life. If this feasibility study is successful, the study team will explore a larger phase III study to determine if the medicines given in the feasibility trial are better or at least the same as standard of care, exercise regimens.
Prostate Liquid Study (PLiS)
Evaluation of chemical elements content in semen and expressed prostatic secretions in the diagnosis and characterisation of prostate cancer.
- More about PLiS
A biomarker is a molecular substance that is an indicator of a biological condition. Cambridge Oncometrix believe that a semen biomarker may be able to predict the presence of prostate cancer. This study will allow work to be carried out on semen samples donated by men who have been identified by their GPs as having a risk of prostate cancer. Normally, GPs refer men with symptoms or a raised PSA to hospital to have an MRI scan and a biopsy.
The PLiS research team at the hospital, will contact potential patients, who have been referred by their GPs, before they have an MRI or a biopsy, ask them if they are willing to be part of the study, consent them and ask them to provide a semen sample. Semen samples will be produced by the potential participants at home into a seminal fluid collection container, they will also complete a study questionnaire. Both the sample and questionnaire will be returned to the central laboratory in Cambridge using the stamped address envelope provided.
In addition to providing a semen sample and completing a questionnaire, each participant will have two blood tests to measure male hormones. Participants then exit the trial, and their usual care carries on from this point.
The PLiS study will register 400 men and of these it is hoped that 300 of them will be able to a provide a semen sample.- Sponsor: University College London (UCL)
- Funder: Cambridge Oncometrix Ltd (Haverhill, UK)
ProRAFT: Radiofrequency Ablation Focal Treatment
A Prospective Development Study evaluating Focal Therapy using Encage™ coiled bipolar radiofrequency ablation in Men with Localised Prostate Cancer (closed).
- More about ProRAFT
Current treatments for early prostate cancer use radiation or surgery to treat the whole prostate. This can cause damage to surrounding structures that control erections and urine flow and can also damage the back passage. This leads to leakage of urine, poor sexual function and back passage bleeding, discomfort, and diarrhoea.
The group has shown that treating only the areas of cancer with heat, cold, or laser-light, can lead to a low rate of these side-effects. The cancer control rates, however, can vary and are not consistent.
The group believes they can do better by using a different type of technology called Encage™, a coiled radiofrequency based bipolar device. This new type of treatment might be more precise and better controlled for prostate treatment than others the group have used.
If it is found to be of value, this treatment could reduce the burden of side-effects that men currently face.
- Sponsor: University College London
- Funder: TROD Medical, Belgium.
- ClinicalTrials.gov: NCT02294903
- ISRCTN: ISRCTN10434678
Publications
Orczyk C., Brew-Graves C., Williams N., Potyka I., Ramachandran N., Freeman A., Emberton M., Ahmed H.U. (2017). Prostate radiofrequency ablation focal treatment (PRORAFT): interim results of a prospective development study. Presented at: American Urological Association (AUA) 2017 Annual Meeting.
Orczyk, C., Barratt, D., Brew-Graves, C., Peng Hu, Y., Freeman, A., McCartan, N., Ahmed, H.U. (2021). Prostate Radiofrequency Focal Ablation (ProRAFT) Trial: A Prospective Development Study Evaluating a Bipolar Radiofrequency Device to Treat Prostate Cancer. J Urol, 101097JU0000000000001567.
PulMiCC
Investigating the value of pulmonary metastasectomy (surgery to remove lung metastases) in patients who have been successfully treated for colorectal cancer.
- More about PulMiCC
Patients who have been treated successfully for bowel cancer (colorectal cancer) sometimes go on to develop nodules of disease in another part of the body. If this disease is found to be related to the original cancer, it is called a metastasis. Some patients develop one or more metastases particularly in the lungs or the liver.
There is a growing trend to remove lung metastases with an operation in the belief that this will help patients live longer. However, there have not been any scientific studies to prove this. There is also very little published information about the side effects of this surgery and how it affects subsequent daily living.
PULMICC is a randomised controlled trial to investigate the value of pulmonary metastasectomy (surgery to remove lung metastases) in patients who have been successfully treated for colorectal cancer.
There is a two-stage consent and randomisation process. Firstly, patients will be invited to consent to having a full range of investigations to assess their suitability for surgery. If found to be suitable, they will be invited to consent to randomisation between active monitoring of their disease or active monitoring with pulmonary metastasectomy.Patients will be followed up regularly for five years to assess their disease status and to measure their quality of life and lung function.
The PulMiCC Trial is now closed to recruitment. Patients are still being followed up.
Publications
Cardillo, G., Mokhles, S., Williams, N., Macbeth, F., Russell, C., Treasure, T. (2015). Comment on: 'KRAS and BRAF mutations are prognostic biomarkers in patients undergoing lung metastasectomy of colorectal cancer.' Variation in survival associated with proto-oncogenes is not evidence for effectiveness of lung metastasectomy. British Journal of Cancer, 113 (11), 1636.
Treasure, T., Williams, N.R. (2017). Best available evidence related to clinical benefit of surgical resection in multimodality treatment of metastatic colorectal cancer indicates that a randomised controlled trial is warranted. European journal of cancer, 75 310-312.
Treasure, T., Farewell, V., Macbeth, F., Batchelor, T., Milosevic, M., et al. (2021). The Pulmonary Metastasectomy in Colorectal Cancer (PulMiCC) burden of care study: Analysis of local treatments for lung metastases and systemic chemotherapy in 220 patients in the PulMiCC cohort. Colorectal Disease, 23 (11), 2911-2922.
Treasure, T., & Williams, N.R. (2021). Lung metastasectomy for colorectal cancer in the PulMiCC randomised controlled trial. The Lancet Regional Health - Europe, 3.
Williams, N. R., Patrick, H., Fiorentino, F., Allen, A., Sharma, M., Milosevic, M., Treasure, T. (2022). Pulmonary Metastasectomy in Colorectal Cancer (PulMiCC) randomized controlled trial: a systematic review of published responses. European Journal of Cardio-Thoracic Surgery, 62 (1).
Milosevic, M., & Treasure, T. (2020). Pulmonary Metastasectomy in Colorectal Cancer (PulMiCC): Updated analysis of 93 randomised patients - control survival is much better than previously assumed. Colorectal Disease.
Williams, N.R., Macbeth, F., & Treasure, T. (2020). Pulmonary metastasectomy in colorectal cancer: PulMiCC and future trials. Quantitative Imaging in Medicine and Surgery, 10 (11), 2215-2217.
Williams, N.R., Milosevic, M., Treasure, T. & Macbeth, F. (2020). The PulMiCC Trial Provides Control Data for Colorectal Lung Metastases Amenable to Local Treatments. Cardiovascular and Interventional Radiology, 44 (4), 654-655.
Williams, N.R., Treasure, T. & Macbeth, F. (2022). The Pulmonary Metastasectomy in Colorectal Cancer (PulMiCC) trial results cast doubt upon benefit of lung and liver metastasectomy at the time colorectal resection. Langenbecks Archives of Surgery, 407 (2), 881-883.
Treasure, T., Williams, N.R. & Macbeth, F. (2022). The full cohort of 512 patients and the nested controlled trial in 93 patients in the Pulmonary Metastasectomy in Colorectal Cancer (PulMiCC) study raise doubts about the effective size at present claimed. Journal of Cardiothoracic Surgery, 17 (1), ARTN 9.
Williams, N.R., Treasure, T., Macbeth, F., & Fallowfield, L. (2021). The Prospective Observational Cohort and the Nested Randomized Controlled Trial in the Pulmonary Metastasectomy in Colorectal Cancer (PulMiCC Study) Question the Reliance on Existing Evidence for the Magnitude of Benefit From Lung Metastasectomy. American Journal of Clinical Oncology-Cancer Clinical Trials, 44 (9), 502-503.
Treasure, T., Farewell, V., Macbeth, F., Williams, N.R., Brew-Graves, C., et al. (2019). Pulmonary Metastasectomy versus Continued Active Monitoring in Colorectal Cancer (PulMiCC): a multicentre randomised clinical trial. Trials, 20 (1), ARTN 718.
Radiocyst
Investigating if radiofrequency ablation can be used to destroy small cystic tumours of the pancreas and collecting health economics data of the cost of treatment.
- More about Radiocyst
Study title: A Phase II multicentre trial of endoscopic ultrasound guided radiofrequency ablation of cystic tumours of the pancreas.
Up to 13.5% of patients that undergo MRI scans of their abdomen without pancreatic symptoms are found to have an incidental pancreatic cyst, with the frequency increasing with age.
Premalignant pancreatic cysts may be indolent for several years before malignant transformation, creating a window of opportunity for minimally invasive intervention and cure. Observation is associated with significant anxiety for patients and a growing cost to the National Health Service. Surgery for this usually benign condition is associated with not insignificant morbidity and mortality. New early treatment options for premalignant tumours are urgently required.
New catheters for treating cystic tumours of the pancreas during endoscopy have been developed. Studies in animals and in a small number of patients have suggested that radiofrequency ablation is a safe and effective tool for treating growths in the pancreas.
This study will evaluate if radiofrequency ablation can be used to destroy small cystic tumours of the pancreas (so patients do not require long-term follow up) and collect some health economics data (assess cost of treatment).
It will also evaluate the safety and efficacy of a novel minimally invasive technique for the treatment of pancreatic cystic tumours; endoscopic ultrasound guided radiofrequency ablation (EUSRFA). If successful, it will offer an alternative to long term observation or surgery for patients with this condition.- Sponsor: University College London
- Funder: NiHR RfPB
- ClinicalTrials.gov Identifier: NCT02343692
Re-IMAGINE
Re-IMAGINE aims to enable better prediction of prostate cancer status without biopsy and which prostate cancers are likely to progress over time.
- More about Re-IMAGINE
One in eight men will develop prostate cancer. The risk increases with age. Some will die of it. Yet, our ability to identify men whose cancer will decrease either their quality or quantity of life remains poor. Nowhere else in modern medicine are the errors of overdiagnosis, overtreatment, missed diagnoses and poor risk-stratification more extreme. These four errors - which directly impact the one million men who undergo prostate biopsy each year in Europe alone - result in treatments with little to no benefit. They leave men with significant life-long harms and waste valuable resources.
The Re-IMAGINE Consortium looks for discoveries to correct these key errors. The project combines the underlying molecular changes in the cancer with state-of-the-art imaging, enabling predictions of prostate cancer status for the individual (low, medium, or high risk) without recourse to biopsy. It will also allow prediction of which prostate cancers are likely to progress over time and which are not, this has largely eluded scientists so far. As a result, men will be subject to fewer but better biopsies, improved risk stratification, appropriate treatment allocation, more benefit, less harm, and more cost-effective care.
Re-IMAGINE builds on established partnerships between patients, advocacy organisations, clinicians, imaging experts, molecular biologists, methodologists, and a broad range of industrial partners. The vision of Re-IMAGINE is built on recently published work showing that magnetic resonance imaging (MRI) of the prostate was 100% better at identifying men at risk compared to the standard prostate biopsy, and moreover missed no men with potentially fatal disease. MRI is now certain to become the future cornerstone of the risk-stratification process for men at risk of early prostate cancer. However, little is known about the use of MRI in combination with other markers in the body.
The Re-IMAGINE programme consists of several work-strands. WS1 will create the first group (cohort) of men who receive a prostate cancer diagnosis by means of an MRI-based pathway. These men will, after appropriate consent, donate some tissue, blood, and urine for marker analysis. They will be recruited from several major centres in London (about three initially), that have high quality MRI in place. WS2 will test the performance of MRI in a population where cancer is less common - in men who have not had a PSA test. This will allow us to predict the exact proportion of men at risk within the community.These work streams will generate a lot of data (clinical information, imaging data, as well as information from markers in blood, tissue, and urine). We will combine all this data for each patient using a secure database, in line with the General Data Protection Regulations (GDPR), analysis will include mathematical techniques.
Such a complex project will require high levels of project management and coordination. This will be achieved by building on existing and fruitful collaborations. The Re-IMAGINE programme will have a strong PPI work strand.
Re-IMAGINE will transform the management of early prostate cancer from an era in which imprecision and uncertainty is the norm to one in which any patient can expect and benefit from a precise prediction of his own risk and better care as a result.
SpectraCure P18
Open-label clinical study of safety and assessment of adequacy of effectiveness of the SpectraCure P18 System (Interstitial multiple diode lasers and IDOSE® software) and verteporfin for injection (VFI) for the treatment of recurrent localized prostate cancer.
- More about SpectraCure

Following radiotherapy, surgical removal of the prostate is more difficult to do and comes with significant risk of associated side effects. These include urinary incontinence, erectile dysfunction, and rectal damage.
SpectraCure P18 aims to establish the safety and effectiveness of a new system for delivering photodynamic therapy (PDT) for prostate cancer, which can monitor the effect of treatment and modify the treatment dose. PDT uses light from a laser to activate a drug which has been injected into the bloodstream, to destroy cancer cells. The procedure is done under general anaesthetic.The study will determine the correct light threshold dose and the lowest effective drug dose. Although the drug Verteporfin is widely used in the treatment of other conditions, this is the first time that it is being used in the treatment of prostate cancer.
PDT is an established therapy for certain types of superficial cancers such as skin cancer. Interstitial photodynamic therapy (IPDT) is a development of this technique for solid and deeper lying cancers such as prostate cancer. The light is delivered to the tumour via optical fibres. The light causes the photosensitizing drug to react with oxygen in the tissue to produce an active form of oxygen which causes cell death in the targeted area. Neither the photosensitizer nor the light or tissue oxygen exert any effect until they are combined.
- Study number: SPC11-01-110
- Sponsor & Funder: Spectracure AB, Sweden
- ClinicalTrials.gov Identifier: NCT03067051
TARGIT A
The TARGIT-A randomised clinical trial of Intrabeam TARGIT IORT delivered in a single dose during lumpectomy compared to external beam radiotherapy delivered over weeks.
- More about TARGIT A
About 70% of patients with breast cancer are eligible for breast-conserving surgery (a lumpectomy), after which the remaining breast is treated with radiotherapy. This avoids a full mastectomy.
Traditionally, external beam radiotherapy (EBRT) is delivered to the entire breast in small doses every day for 3-6 weeks, requiring patients to travel to and from the radiotherapy centre every working day. This can be impractical and strenuous.
The TARGIT (TARGeted Intraoperative radioTherapy or IORT) procedure precisely delivers radiation in a single dose during the lumpectomy operation over 15-35 minutes, using a ball-shaped device that is placed in the space where the tumour was.
This prevents unnecessary potentially harmful radiation to healthy tissues (skin, heart, lungs, etc.) and the areas nearest to the tumour site receive the most radiation. In this way, four-fifths of patients avoid EBRT altogether.
The TARGIT-A (TARGeted Intraoperative radioTherapy Alone) trial compared Intrabeam TARGIT IORT with EBRT in 3451 patients who were aged ≥45 years. It found that:- When TARGIT is given with lumpectomy, the control of breast cancer is much the same as with EBRT.
- The chances of being alive without return of cancer in the breast at five years were 93.9% with TARGIT during lumpectomy and 92.5% with EBRT.
- TARGIT had fewer side effects and fewer deaths from heart attacks or other cancers.
- TARGIT IORT would be less expensive than EBRT, potentially saving the NHS up to £9.1 million a year, without considering the cost savings to patients.
Targeted intraoperative radiotherapy IORT during lumpectomy is an effective, safer, and less expensive option for eligible patients.
Publications
Vaidya, J.S., Bulsara, M., Wenz, F., Joseph, D., et al. (2015). Pride, Prejudice, or Science: Attitudes Towards the Results of the TARGIT-A Trial of Targeted Intraoperative Radiation Therapy for Breast Cancer. International Journal of Radiation Oncology Biology Physics, 92(3), 491-497.
Coombs, N.J., Coombs, J.M., Vaidya, U.J., Singer, J., et al. (2016). Environmental and social benefits of the targeted intraoperative radiotherapy for breast cancer: data from UK TARGIT-A trial centres and two UK NHS hospitals offering TARGIT IORT. BMJ OPEN, 6 (5).
Vaidya, J.S., Wenz F., Bulsara M., Brew-Graves C., et al. (2016). An international randomised controlled trial to compare TARGeted Intraoperative radioTherapy (TARGIT) with conventional postoperative radiotherapy after breast-conserving surgery for women with early-stage breast cancer (the TARGIT-A trial). Health Technology Assessment.
Keshtgar, M., Williams, N.R. (2016). Intraoperative radiation therapy deserves to be made more readily available to patients. Chinese Journal of Cancer Research, 28 (4), 461-462.
Vaidya, A., Vaidya, P., Both, B., Brew-Graves, C., Bulsara, M. & Vaidya, J.S. (2017). Health economics of targeted intraoperative radiotherapy (TARGIT- IORT) for early breast cancer: a cost- effectiveness analysis in the United Kingdom. BMJ Open.
Williams, N.R., Kurylcio, A., Jankiewicz, M., Romanek, J., et al. (2017). Aesthetic outcome after breast conserving surgery and either intraoperative radiotherapy or whole breast external beam radiotherapy for early breast cancer: Objective assessment of patients in a randomized controlled trial in Lublin, Poland. European Journal of Gynaecological Oncology, 38 (6), 867-870.
TARGIT B
An international randomised controlled trial to compare targeted intra-operative radiotherapy boost with conventional external beam radiotherapy boost after lumpectomy for breast cancer in women with a high risk of local recurrence.
- More about TARGIT B

DESIGN: A multi-centre randomised trial to test whether Targeted Intraoperative Radiotherapy as a tumour bed boost (TARGIT-B) is superior in terms of local relapse within the treated breast compared with standard post-operative external beam radiotherapy boost in women undergoing breast conserving therapy who have a higher risk of local recurrence. Patients can be entered before the primary surgery or in a smaller proportion of cases, post-pathology, when a second procedure would be required.
SETTING: Specialist breast units in UK, USA, Canada, Australia, and Europe. 31 centres are recruiting in the TARGIT-A trial, and several are ready to join.
TARGET POPULATION: Breast cancer patients suitable for breast conserving surgery, but with a high risk of local recurrence. The patients should be younger than 45, or, if older, should have certain pathological features that confer a high risk of local recurrence of breast cancer.
TECHNOLOGIES ASSESSED. The TARGIT Technique: The Intrabeam® (Carl Zeiss, FDA approved, and CE marked) is a miniature electron beam-driven source which provides a point source of low energy X-rays (50kV maximum) at the tip of a 3.2mm diameter tube. The radiation source is inserted into the tumour bed immediately after excision of the tumour and switched on for 20-35 minutes to provide intra-operative radiotherapy accurately targeted to the tissues that are at highest risk of local recurrence.
The physics, dosimetry and early clinical applications of this soft x-ray device have been well studied. For use in the breast, the technique was first developed and piloted at University College London. The radiation source is surrounded by a spherical applicator, specially designed (and available in various sizes) to produce a uniform field of radiation at its surface, enabling delivery of an accurately calculated dose to a prescribed depth. It is inserted in the tumour bed and apposed to it with surgical sutures and/or other means. As the x-rays rapidly attenuate, the dose to more distant tissues is reduced. This also allows it to be used in standard operating theatres. 20 Gy is delivered to the tumour bed surface in 20-35 minutes, after which the radiation is switched off, the applicator removed, and the wound closed in the normal way.
This simple technique has potentially several advantages over conventional external beam radiotherapy, interstitial implantation of radioactive wires or conformal external beam radiotherapy. The first pilot of twenty-five cases was at performed at UCL using TARGIT technique as a replacement for the boost dose of radiotherapy. Full dose external beam treatment was subsequently given. The phase II study of 300 patients was published and recently updated with long term data along with favourable toxicity and cosmetic outcome results of individual cohorts.
A mathematical model of TARGIT developed recently (funded by Cancer Research UK) suggests that it could be superior to conventional radiotherapy. Translational research has found that TARGIT impairs the surgical-trauma-stimulated proliferation and invasiveness of breast cancer cells. This effect of radiotherapy may act synergistically with its tumoricidal effect yielding a superior result. This technology has already been assessed (HTA Grant 07/60/49) and the first results were recently fast-tracked and published in The Lancet. We found that in low-risk patients, TARGIT yields a non-inferior outcome.
MEASUREMENT OF COST AND OUTCOME: Patient assessments will be by clinical examination (six-monthly for three years then yearly for 10 years) and mammography (yearly) with ultrasound (if needed). Primary outcome: histologically/cytologically proven local recurrence. Secondary: site of relapse in the breast, overall survival, local toxicity (graded according to RTOG and LENT SOMA criteria), cosmesis, patient satisfaction and health economics (separate protocols).
VIDS: Jayant / Marcelle Bernstein
TARGIT R
Initiation and maintenance of a registry database of patients treated with targeted intraoperative radiation therapy using Intrabeam (TARGIT) following breast conserving surgery for early breast cancer.
- More about TARGIT R

Breast cancer is the most common cancer in the UK. With the introduction of the breast screening programme, many patients are seen in early stages of diagnosis. These are all currently subjected to standard radiotherapy, which is given several months after surgery and would normally consist of a 3–5-week course of daily radiotherapy to the whole breast, as well as a shorter 5–8-day course to the tumour bed.
Results from the TARGIT-A randomised controlled trial in low-risk women over 45 years showed that giving a single dose of radiation with the TARGIT technique at the time of operation gave similar results as a several-week course of radiotherapy.
Studies show that breast cancer patients are given or choose to have their breast removed completely (mastectomy). Reasons include being unable to afford travel to their nearest radiotherapy centre or 15-25 days away for daily treatment. In some cases, blindness, previous external beam radiotherapy, claustrophobia, or age preclude these patients from having external beam radiotherapy.
In such cases, some we would like to collect this data to enhance future decisions regarding best treatment practices for these subgroups of patients.
The TARGIT R (registry) study will gather further information on the technique in a much more diverse population with early breast cancer. The primary objective is to monitor safety and toxicity on patients treated outside of a randomised controlled trial.
Careful monitoring of the data will enable us to see early indications of subgroups of patients, or clinical teams, which show higher than expected rates of local recurrence (effectiveness) Cost effectiveness data and long-term cardiopulmonary events will also be collected as secondary endpoints.
- Sponsor: University College London (UCL)
- ISRCTN Number: ISRCTN91179875
- Clintrial.gov Number: NCT02947425
TARGIT X
Extended follow-up of TARGIT-A to collect more data about the health status of all patients and find out about longer-term differences in the effects of these treatments on health.
- More about TARGIT X
All UK patients who participated in the TARGIT-A Trial were initially treated for early breast cancer between 2000-2012.
A total of 3,451 patients from 33 hospitals in 11 countries participated in the trial. Traditional radiotherapy given over weeks (external beam radiotherapy, EBRT) was compared with TARGeted Intraoperative radioTherapy (TARGIT-IORT) as a single dose given during the operation to remove the breast cancer.
The trial was funded by the Health Technology Assessment (HTA) programme of the Department of Health, UK and sponsored by University College London. The results from this trial have been published in major medical journals and have already started changing the way breast cancer in treated around the world.
The trialists wish to continue collecting data about the health status of all patients to find out about longer term differences in the effects of these treatments on health. An analysis of this information could improve treatment for patients with breast cancer. For this, HTA have granted further funding.
- Supportor: NIHR HTA grant number: 14/49/13
- Sponsor: University College London (UCL)
- Protocol version: Version 1.0 10Nov17
- R&D / Sponsor Ref: 17/0774
TOXYC
A randomised controlled trial of targeted oxygen therapy in mechanically ventilated critically ill patients (a feasibility study).
- More about TOXYC

The administration of high concentrations of oxygen to patients have proved to be harmful in some settings, especially in patients already suffering from damage to their lungs. Patients who require breathing assistance with an artificial ventilator due to disease of their lungs are frequently given high concentrations of oxygen to maintain a normal level of oxygen in their blood. It is therefore essential to strike a balance between the benefits and harms of having a normal blood oxygen level.
We intend to recruit critically ill patients requiring artificial ventilation into a randomised controlled trial to assess the feasibility of conducting a trial that determines blood oxygen levels. Patients will be allocated to either a normal or lower than normal blood oxygen group. Doctors and nurses looking after patients in the trial will adjust the amount of oxygen, they administer to ensure that the patient's blood oxygen level remains in the allocated target range. Blood oxygen level will be monitored with a standard non-invasive monitor (a pulse oximeter). A total of 60 patients will be recruited at two different hospitals.
Feasibility will be assessed by the ease of recruiting complex critically ill patients into a trial of this nature, and the ability of clinical teams to deliver the intervention. A secondary purpose is to look at specific biological markers in blood samples collected from participants, to see if they are associated with clinical outcomes.
The information from this study will be used to create a large multi-centre trial to fully evaluate targeted oxygen therapy in critically ill patients.
- Sponsor: University College London (UCL)
- Funders: The National Institute for Health and Research (Research for Patient Benefit), The Royal Free Charity
- Clinicaltrial.gov: NCT03287466
Publications
D.S. Martin, C. Brew-Graves, N. McCartan, G. Jell, I. Potyka, J. Stevens, N. Williams, M. McNeil, B.R. O'Driscoll, M. Mythen, M.P.W. Grocott, (2019). Protocol for a feasibility randomised controlled trial of targeted oxygen therapy in mechanically ventilated critically ill patients. BMJ Open
True NTH UK (Post-Surgical Follow Up)
Prostate cancer is the most common cancer in men in the UK, with over 40,000 new diagnoses every year. Earlier cancer detection and effective treatments are contributing to increasing survival rates. Radical prostatectomy is a commonly performed operation to treat localised prostate cancer.
- More about True NTH
Prostate cancer is the most common cancer in men in the UK, with over 40,000 new diagnoses every year. Earlier cancer detection and effective treatments are contributing to increasing survival rates. Radical prostatectomy is a commonly performed operation to treat localised prostate cancer.
Men who choose radical surgery for prostate cancer can experience side effects, including urine leakage and problems with erections. Generally, men would like to have a greater understanding of, and support for, these side effects, both before they choose a treatment and when they are dealing with side effects after treatment.
One of the difficulties in discussing the possible side effects after prostate cancer surgery is that they will depend upon the urinary and sexual function that a man has prior to the operation, as well as on the details of the operation itself, which is influenced by the location and aggressiveness of the prostate cancer.
The True NTH UK Post-Surgical Follow Up Programme focuses on using patient reported outcome measures (PROMs) to assess the extent and timeline for recovery of urinary and sexual function after radical surgery for prostate cancer.
A new instrument will be developed for radical prostatectomy that can be used with men in the UK to monitor their recovery in the first 12 months after surgery. It is envisaged that the PROMs data will be used in clinical practice to monitor progress in outcomes for individual patients. The programme will also allow a comparison of results of surgeons and hospitals against appropriate benchmarks for urinary and sexual outcomes after radical prostatectomy.
Patients will need to complete an online questionnaire before they have their surgery, and at 1, 3, 6 and 12 months after their radical prostatectomy surgery (5 times in total). This questionnaire will ask about participant’s symptoms and how they feel about them. It will ask about their urinary, bowel and sexual function, and overall health.The TrueNTH trial has two phases. Phase I consists of evaluating the US developed monitoring tool (MSK instrument) and using focus groups to gather the opinions of UK men. Using this feedback, adjustments were made to the reporting tool. Patients recruited into Phase II use the adapted tool to assess the robustness at an individual patient level.
Phase I: 429 patients were recruited across 9 sites. Phase 1 is complete.
Phase II: We aim to take the whole cohort up to 3000 patients across approximately sites. Phase II is still recruiting, with new sites still able to join the study.
- Sponsor: University College London
- Funder: Prostate Cancer UK
Publications
Evans S.M., Millar J.L., Moore C.M., et al. Cohort profile: the TrueNTH Global Registry - an international registry to monitor and improve localised prostate cancer health outcomes. BMJ Open 2017;7:e017006.
Sampurno, F., Zheng, J., Di Stefano, L., Millar, J. L., Moore, C. (2018). Quality Indicators for Global Benchmarking of Localized Prostate Cancer Management. The Journal of Urology.
Sampurno F, Cally J, Opie J.L., Moore, C. et al. Establishing a global quality of care benchmark report. Health Informatics Journal. 2021;27(2).
Tumouroids
Acceptability and feasibility study of patient-specific 'tumouroids' as personalised treatment screening tools.
- More about Tumouroids
In England, more than three hundred thousand people are diagnosed with cancer each year. The diagnostic and treatment pathways for multiple cancers have greatly developed over the past decade. However, novel treatments are expensive and currently discrimination between responders and non-responders is still suboptimal. There is a pressing need to develop tools that allow for better disease characterisation and stratification. Personalised medicine, whereby prevention, diagnosis, and treatment of diseases is aimed at the individual level, is a growing field.
Predicting patient-specific treatment response is challenging as response depends not only on the characteristics of cancer cells but also on how these cells interact with their immediate surrounding environment and on how the tumour interacts with the host. A simplistic model is therefore insufficient to predict treatment response.
Complex, patient-derived animal models have been used for this effect but are expensive, may take up to six months to provide clinically relevant answers, and pose ethical issues. Historically, in vitro models lacked complexity as they were based solely on the two-dimensional (2D) growth of cancer cells. Nowadays the use of 3D tumour models has provided an extra level of complexity to in vitro studies. With these models it is possible to recreate tumour characteristics that were lost in 2D, such as cell-cell interaction between cancer cells and between cancer and stromal cells, cell-matrix interaction, or hypoxia.
We have developed a 3D complex tumour model named tumouroid. Using this, we have undertaken preliminary work that has allowed us to grow patient-derived tumouroids using primary cancer cells from patients. This personalised platform can be challenged by therapeutics used in clinical practice and response to treatment can be assessed via appropriate assays.
Our goals are twofold:
- To assess patient acceptability to the use of patient derived tumour models for future treatment decision.
- To assess the feasibility of generating patient specific renal cancer tumouroids to be used as platforms to test drug response.
- Sponsor: University College London (UCL)
- Funder: National Institute of Health Research i4i Invention for innovation grant
- Clinicaltrial.gov: NCT03300102
More about our work in Tumouroids
Publications
Stamati, K., Neves, J.B., De Albuquerque Garcia Redondo, P., Emberton, M., et al. (2019). Feasibility of establishing renal cancer patient-specific 'tumouroids' as personalised treatment screening tools.
Tran, M., Neves, J., Stamati, K., Redondo, P., Brew-Graves, C., Emberton, M., et al. (2019). Acceptability and feasibility study of patient-specific 'tumouroids' as personalised treatment screening tools: protocol for prospective tissue and data collection of participants with confirmed or suspected renal cell carcinoma. International Journal of Surgery Protocols.
 Close
Close

