Laszlo Hosszu
 l.hosszu@prion.ucl.ac.uk • PubMed • |
Research Synopsis
NMR spectroscopy is a unique method of studying proteins at the atomic level in solution, allowing us to determine their structure, function, and how they fold and unfold from their normal biological state. Understanding this is of major biomedical importance as a number of neurodegenerative diseases, such as Alzheimer’s, Parkinson's and the prion diseases, CJD and BSE, involve protein misfolding and partially and misfolded protein states. We have shown how NMR can be used to determine structural and dynamic information of such states, which provide a basis for identifying where and how to interrupt amyloidogenesis before the onset of neurodegeneration.
Recent highlights include the structure determination and biophysical characterisation of the disease-resistant form of prion protein, V127, in its normal cellular fold. Key regions of the protein which are associated with the development of prion diseases were identified, providing insights into its protective mechanism, and how prion protein misfolding is involved.
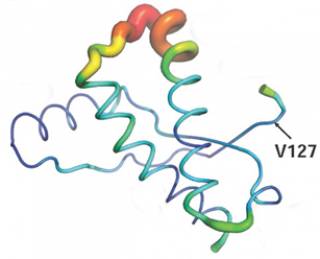 |
Structural variability in V127 prion protein |
Structural perturbations induced by protective V127 mutation in cellular prion protein
Hosszu LLP, Conners R, Sangar D, Batchelor M..... McAuley K, Brady RL, Bieschke J, Waltho JP, Collinge J (2018)
A novel protein variant in a patient with semantic dementia
Kenny J, Woollacott I, Koriath C, Hosszu LLP, Adamson G, Rudge P..... Mead S, (2017) . J. Neurology, Neurosurgery, & Psychiatry, 88, 890-892
Ex vivo mammalian prion are formed of paired double helical prion protein fibrils
Terry C, Wenborn A, Gros N, Sells J, Joiner S, Hosszu LLP..... Wadsworth JD (2016) . Open Biology, 6, 160035
N-terminal domain of prion protein directs its oligomeric association
Trevitt CR, Hosszu LLP, Batchelor M, Panico S, Terry C..... Collinge J, Waltho JP, Clarke AR (2014) Journal of Biological Chemistry 289, 25497-508
The H187R mutation of the human prion protein induces conversion of recombinant prion protein to the PrP(Sc)-like form.
Hosszu LLP, Tattum MH, Jones S, Trevitt CR, Wells MA, Waltho JP, Collinge J... Clarke AR (2010) Biochemistry, 49, 8729-38
Folding kinetics of the human prion protein probed by temperature jump
Hart T, Hosszu LLP, Trevitt CR, Jackson GS, Waltho JP, Collinge J, Clarke AR (2009). Proc. Natl. Acad. Sci .USA, 106, 5651-6
Location and properties of metal-blinding sites on the human prion protein
Jackson GS, Murray I, Hosszu LLP, Gibbs N, Waltho JP, Clarke AR, Collinge J (2001) Proc. Natl. Acad. Sci .USA. 98, 8531-5
Structural mobility of the human prion protein probed by backbone hydrogen exchange
Hosszu LLP, Baxter NJ, Jackson GS, Power A, Clarke AR, Waltho JP, Craven CJ, Collinge J (1999) . Nat. Struct. Biol. 6, 740-3
 Close
Close

