Jonathan Wadsworth
Research Synopsis
Prions are infectious agents causing a group of closely related lethal brain diseases that include scrapie in sheep, BSE in cattle, CWD in deer and elk and various forms of CJD in humans. Unlike all other infectious agents (bacteria and viruses) the infectious particle does not contain genetic information (genes) but instead consists of pathogenic assemblies of prion protein (PrP) which replicate in the central nervous system leading to progressive neurodegeneration and eventually death of the infected host.
Although prions do not carry genetic material they come in several different forms, called prion strains which are responsible for different forms of the disease in humans and animals. Our research aims to define the fundamental biology of what makes prion strains different from one another why some are able to cross from animals to humans to cause disease.
Recent work in the Unit using state-of-the-art electron cryo-microscopy (cryo-EM) with colleagues at Birkbeck College has now revealed prion structures at near-atomic resolution and has shown that infectious prion fibrils from distinct mouse prion strains, called ME7 and RML, are built from distinctly folded chains of PrP. The structure of the RML prion fibril is published at Nature Communications and the ME7 structure published at Nature Chemical Biology, with its detailed side-by-side comparison with the RML structure.
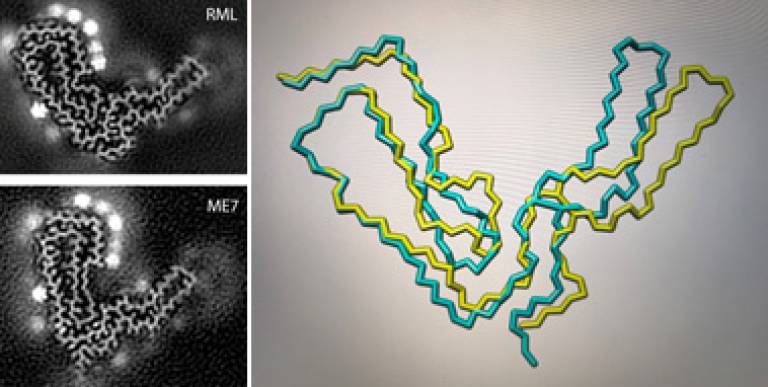
Cross-sections of infectious prion fibrils from RML and ME7 mouse prion strains.
Figure courtesy of Dr Szymon Manka.
Methods we have now established to reveal structural differences among mouse prion strains can now be applied to explore similarities between different human and animal prion strains. Overall we aim to establish a comprehensive structure-based classification system and use this to evaluate which existing or newly emerging animal prion strains might pose a threat to humans. This new information should have direct translation to protecting public health.
A structural basis for prion strain diversity
Szymon W. Manka, Adam Wenborn, Jemma Betts, Susan Joiner, Helen R. Saibil , John Collinge & Jonathan D. F. Wadsworth
Nature Chemical Biology (2023)
Prion strains viewed through the lens of cryo-EM.
Manka SW, Wenborn A, Collinge J, Wadsworth JDF.Cell Tissue Res. 2022 Aug 27. doi: 10.1007/s00441-022-03676-z. Online ahead of print.PMID: 36028585 Review.
2.7 Å cryo-EM structure of ex vivo RML prion fibrils.
Manka SW, Zhang W, Wenborn A, Betts J, Joiner S, Saibil HR, Collinge J, Wadsworth JDF.Nat Commun. 2022 Jul 13;13(1):4004. doi: 10.1038/s41467-022-30457-7.PMID: 35831275 Free PMC article.
Highly infectious prions are not directly neurotoxic
Benilova I, Reilly M, Terry C, Wenborn A, Schmidt C, Marinho AT, Risse E, Al-Doujailya H, Wiggins De Oliveira M, Sandberg MK, Wadsworth JDF, Jat PS, Collinge J. PNAS September 22, 2020 117 (38) 23815-23822; first published September 8, 2020; https://doi.org/10.1073/pnas.2007406117
Structural features distinguishing infectious ex vivo mammalian prions from non-infectious fibrillar assemblies generated in vitro
Terry C, Harniman RL, Sells J, Wenborn A, Joiner S, Saibil HR, Miles MJ, Collinge J, Wadsworth JDF.
Sci Rep. 2019 Jan 23;9(1):376. doi: 10.1038/s41598-018-36700-w.
Ex vivo mammalian prions are formed of paired double helical prion protein fibrils
Terry C, Wenborn A, Gros N, Sells J, Joiner S, Hosszu LL, Tattum MH, Panico S, Clare DK, Collinge J, Saibil HR, Wadsworth JD. Open Biol. 2016 May;6(5). pii: 160035. doi: 10.1098/rsob.160035. Epub 2016 May 4. PMID:27249641
A novel and rapid method for obtaining high titre intact prion strains from mammalian brain
Wenborn A, Terry C, Gros N, Joiner S, D'Castro L, Panico S, Sells J, Cronier S, Linehan JM, Brandner S, Saibil HR, Collinge J, Wadsworth JD.
(2015) Sci. Rep. 5, 10062.
A naturally occurring variant of the human prion protein completely prevents prion disease
Emmanuel A. Asante, Michelle Smidak, Andrew Grimshaw, Richard Houghton, Andrew Tomlinson, Asif Jeelani, Tatiana Jakubcova, Shyma Hamdan, Angela Richard-Londt, Jacqueline M. Linehan, Sebastian Brandner, Michael Alpers, Jerome Whitfield, Simon Mead, Jonathan D.F. Wadsworth and John Collinge. Nature (2015) doi:10.1038/nature14510.
 Close
Close


