Azy Khalili
 a.khalili@prion.ucl.ac.uk Tel: 020 7679 5015 Courtauld Building, Room 501C UCL Profile • PubMed |
|
Research Synopsis
Development of anti-PrP monoclonal antibody and immunotherapy for treatment of human prion disease
Prion diseases, such as CJD are fatal, transmissible, neurodegenerative conditions and their common feature is conversion of normal cellular prion protein (PrPC) into disease related abnormal isoforms. This involves a conformational change from α-helical structure to a rich amyloid β-sheet. PrPC is expressed on most cells particularly brain and immune cells, and the disease related abnormal isoforms accumulate in the brain and the lymphoreticular system, without any antibody being produced against PrP.
Expression of PrPC is essential for prion propagation as no prion disease occurs in PrP knock out mice. Thus, targeting PrPC with ligands like antibodies can prevent its conversion to disease-related formats and halt prion propagation. With the aim of developing a passive immunisation for human prion diseases, we produced a large number of anti-PrP monoclonal antibodies (mAb). Of these, we selected ICSM18 (IgG1) and ICSM35 (IgG2b) for their high affinity binding native PrP and effective neutralisation of prion infectivity.
These mAbs were humanised and after testing, an IgG4 isotype of ICSM18 was selected as the clinical candidate. Both murine and humanised ICSM18 bind native PrP with nM affinity for PrPC, can neutralise prion infection in vitro and in vivo and significantly extend survival time of prion-infected mice. A range of preclinical efficacy, pharmacokinetic and safety studies were carried out for the humanised ICSM18 and its efficacious doses determined.
Clinical immunoassays are developed for the humanised ICSM18 immunotherapy and further investigation. The aim is a safe and efficacious humanised ICSM18 immunotherapy in man.
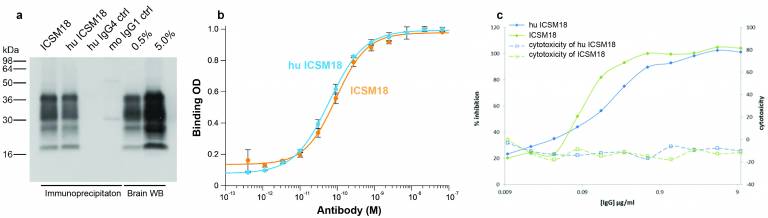 |
ICSM18 and humanised ICSM18 (a) bind all major PrP glycoforms from human (hu) brain homogenate by immunoprecipitation and western blot (WB) with no non-specific binding by hu IgG4 and mouse (mo) IgG1 isotype control (ctrl), (b) show high-affinity interaction to surface-bound recombinant PrP 91-231 by ELISA, (c) inhibit propagation of prions in infected cells in vitro while cytotoxicity is minimal |
Reply to: Intrinsic Toxicity of Antibodies to the Globular Domain of the Prion Protein
Purro SA, Mead S, Khalili-Shirazi A, Nicoll AJ, Collinge J. (2018) Biol Psychiatry 1;84(7):e53-e54.
Peripheral administration of a humanised anti-PrP antibody blocks Alzheimer's disease Aβ synaptotoxicity
Klyubin I, Nicoll AJ, Khalili-Shirazi A, Farmer M, Canning S, Mably A, Linehan J, Brown A, Wakeling M, Brandner S, Walsh DM, Rowan MJ, Collinge J. (2014) J of Neurosci 34:6140–6145
PrP antibodies do not trigger mouse hippocampal neuron apoptosis
Klöhn PC, Farmer M, Linehan JM, O'Malley C, Fernandez de Marco M, Taylor W, Farrow M, Khalili-Shirazi A, Brandner S, Collinge J. (2012) Science 6: 335-52.
Crystal structure of human prion protein bound to a therapeutic antibody
Antonyuk SV, Trevitt CR, Strange RW, Jackson GS, Sangar D, Batchelor M, Cooper S, Fraser C, Jones S, Georgiou T, Khalili-Shirazi A, Clarke AR, Hasnain SS, Collinge J. (2009) Proc Natl Acad Sci U S A. 106:2554-8.
A novel pathogenic pathway of immune activation detectable before clinical onset in Huntington's disease
Björkqvist M, Wild EJ, Thiele J, Silvestroni A, Andre R, Lahiri N, Raibon E, Lee RV, Benn CL, Soulet D, Magnusson A, Woodman B, Landles C, Pouladi MA, Hayden MR, Khalili-Shirazi A, Lowdell MW, Brundin P, Bates GP, Leavitt BR, Möller T, Tabrizi SJ. (2008) J Exp Med. 205:1869-77.
Beta-PrP form of human prion protein stimulates production of monoclonal antibodies to epitope 91-110 that recognise native PrPSc
Khalili-Shirazi A, Kaisar M, Mallinson G, Jones S, Bhelt D, Fraser C, Clarke AR, Hawke SH, Jackson GS, Collinge J. (2007) Biochim Biophys Acta. 1774:1438-50.
Protein conformation significantly influences immune responses to prion protein
Khalili-Shirazi A, Quaratino S, Londei M, Summers L, Tayebi M, Clarke AR, Hawke SH, Jackson GS, Collinge J. (2005) J Immunol. 174:3256-63.
 Close
Close

