Determination of the dissolution and precipitation rates of the major rock forming minerals.
The evolution of geochemical processes in at the surface and shallow subsurface of the Earth is governed to a large extent by the rates of reactions among minerals and their co-existing fluids. Such reactions control to a large extent the mobility of toxic and radioactive waste products, the quality of our drinking water, and our ability to successfully store carbon-dioxide in the subsurface.
Our efforts build on a decades long effort to characterize the rates and mechanisms of the dissolution and precipitation rates of the major rock forming minerals and those minerals essential for the long term-sustainable development of our society. Though these efforts we are developing methods to predict effect of anthropogenic influence on natural systems. Experiments take advantage of both classic closed and flow reactors as well as state-of-the-art near to atomic-scale surface techniques. For example, magnesite (MgCO3) growth rates have been measured directly from step advancement rates using atomic force microscopy in collaboration with the GET laboratory in Toulouse, France and the Mineralogy department of the University of Munich, Germany (Saldi et al. 2009, 2012).
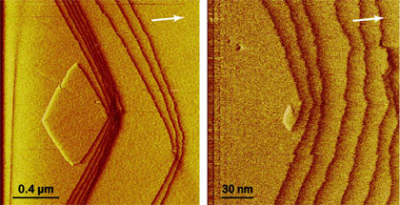 |
| Figure: HAFM images showing spiral growth at screw dislocations on a growing magnesite surface. The image on the right is the detail of one of the centre of nucleation of the image on the left. Experimental conditions: T = 100 _C, X = 47.3. White arrows represent the projection of the c-axis which enters downward into the plane of the page. |
 Close
Close


