New publication by Baum and Saric Labs in BMC Biology
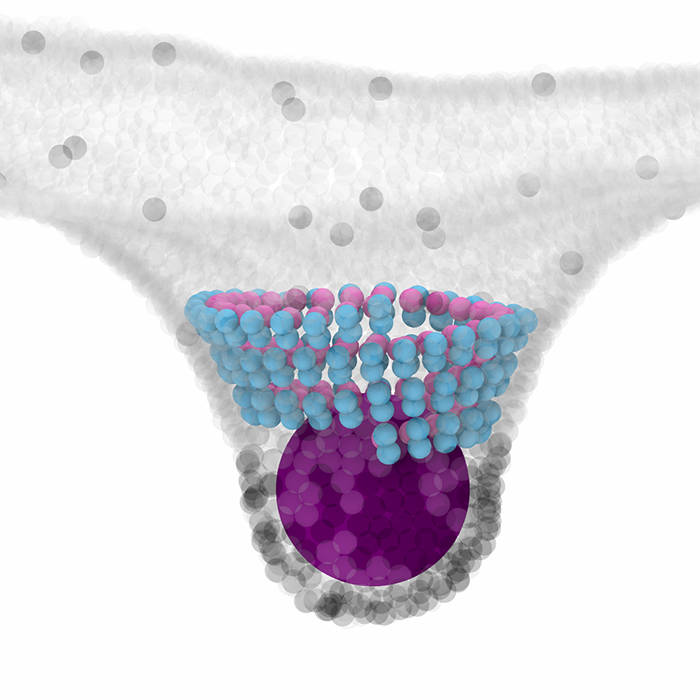
ESCRT-III is a ubiquitous biological nanomachine that reshapes and cuts cell membranes in a wide range of cellular processes, from vesicle formation, HIV and Ebola release, to cell division. A new publication in BMC Biology by the Baum and Saric labs describes the first computer model to capture the physical mechanisms of how the ESCRT-III nanomachinery might operate. They propose that the change in the ESCRT-III filament geometry produces mechanical forces to drive membrane scission, and show that this mechanism can capture all the experimentally reported cases of ESCRT-III driven membrane sculpting, including the formation of downward and upward membrane cones and tubules, and the fission of cargo-containing vesicles.
Abstract from the article:
ESCRT-III is a membrane remodelling filament with the unique ability to cut membranes from the inside of the membrane neck. It is essential for the final stage of cell division, the formation of vesicles, the release of viruses, and membrane repair. Distinct from other cytoskeletal filaments, ESCRT-III filaments do not consume energy themselves, but work in conjunction with another ATP-consuming complex. Despite rapid progress in describing the cell biology of ESCRT-III, we lack an understanding of the physical mechanisms behind its force production and membrane remodelling. Here we present a minimal coarse-grained model that captures all the experimentally reported cases of ESCRT-III driven membrane sculpting, including the formation of downward and upward cones and tubules. This model suggests that a change in the geometry of membrane bound ESCRT-III filaments—from a flat spiral to a 3D helix—drives membrane deformation. We then show that such repetitive filament geometry transitions can induce the fission of cargo-containing vesicles. Our model provides a general physical mechanism that explains the full range of ESCRT-III-dependent membrane remodelling and scission events observed in cells. This mechanism for filament force production is distinct from the mechanisms described for other cytoskeletal elements discovered so far. The mechanistic principles revealed here suggest new ways of manipulating ESCRT-III-driven processes in cells and could be used to guide the engineering of synthetic membrane-sculpting systems.
 Close
Close