New collaborative publication for Saiardi Lab
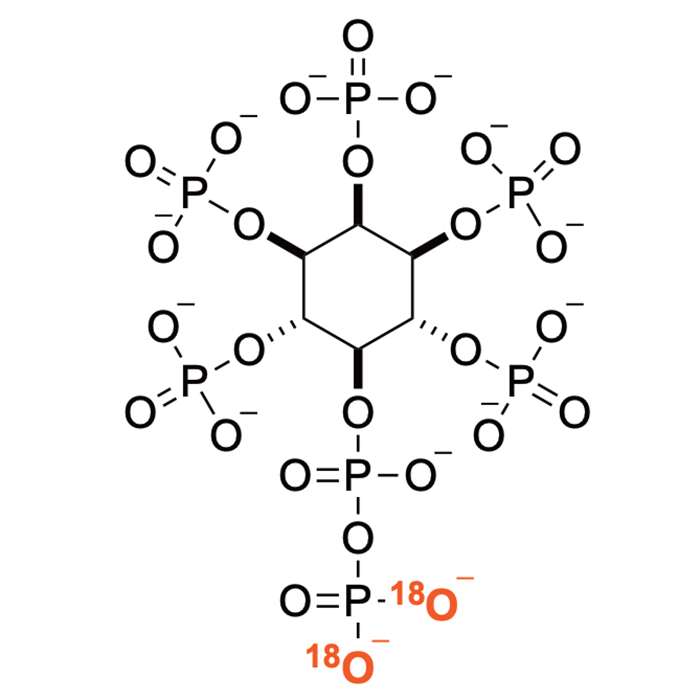
New collaborative publication in Angewandte Chemie International Edition in English for the Saiardi Lab. The development of heavy water-based 18O‑phosphoramidite reagents in the Jessen Lab (University of Freiburg) has allowed the synthesis of heavy isotope analogues of nucleotides, inositol pyrophosphates, and inorganic polyphosphates (polyP). The 18O-labelled inositol pyrophosphates were utilized to assign these metabolites in biological samples utilizing capillary electrophoresis electrospray ionization triple quadrupole mass spectrometry. The use of 18O-isotope labeled inositol phosphates now represent the state-of-the-art in quantitative mass spectrometry for this class of compounds.
Article abstract:
Stable isotope labelling is state-of-the-art in quantitative mass spectrometry, yet often accessing the required standards is cumbersome and very expensive. Here, a unifying synthetic concept for 18O-labelled phosphates is presented, based on a family of modified 18O2‑phosphoramidite reagents. This toolbox offers access to major classes of biologically highly relevant phosphorylated metabolites as their isotopologues including nucleotides, inositol phosphates, -pyrophosphates, and inorganic polyphosphates. 18O-enrichment ratios >95% and good yields are obtained consistently in gram-scale reactions, while enabling late-stage labelling. We demonstrate the utility of the 18O-labelled inositol phosphates and pyrophosphates by assignment of these metabolites from different biological matrices. We demonstrate that phosphate neutral loss is negligible in an analytical setup employing capillary electrophoresis electrospray ionization triple quadrupole mass spectrometry.
 Close
Close