Christina Dix on her recent Developmental Cell paper
Congratulations to the Baum Lab for their publication “The Role of Mitotic Cell-Substrate Adhesion Re-modeling in Animal Cell Division” in Developmental Cell. We spoke to the lead author Christina Dix about the questions this paper addresses, the frustrations of cells wandering out of view during long microscopy sessions and Christina’s new job in the Making Lab at the Crick Institute.
Growing up with scientist parents, Christina heard a lot about science at the dinner table and liked trying to understand how the world works. After enjoying her experiences in the lab as an undergraduate during her course in Biological and Biomedical Sciences at the National University of Ireland Maynooth, Christina chose the PhD programme at the LMCB, as the rotation scheme gave her the opportunity to experience different projects. She joined the Baum Lab and we talked to her about some of the work she carried out during her PhD, which is discussed in this new publication.
Can you tell us about your new paper and the questions you were trying to address?
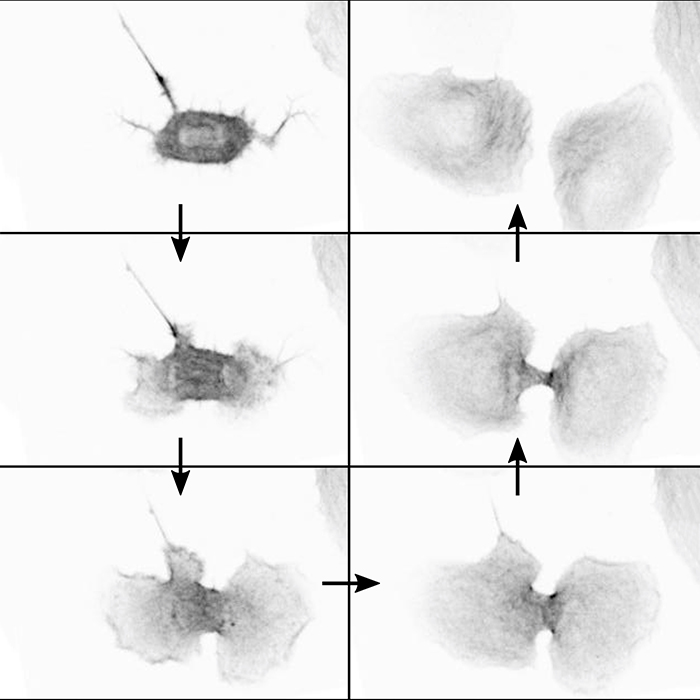
The paper examined how a cell’s adhesion to substrate changes as it goes through mitosis and the effect this has on cell division. When cells enter mitosis, they need to reduce their adhesions to the substrate so that they can round up and become spherical. This is important for them to orientate their spindle properly and correctly separate their DNA. Traditionally it has been thought that during cytokinesis a ring forms between the daughter cells and constricts, squeezing the two cells apart. This does happen, but we found that if we stopped this ring from forming, cells adhere to the substrate and pull apart, showing that these adhesions are also important.
You used a variety of techniques to look into this. Could you tell us a little bit about them?
We primarily used live cell imaging. We made a stable cell line with an adhesion marker and then used this to follow how the cell’s adhesions and shape change during mitosis. We also collaborated with several people to explore different technologies. We carried out a number of experiments using microfabrication. For instance, we created micro-patterns on the substrate cells adhered to, thus confining them and forcing them to be a certain shape, in order to dictate how they were able to divide. We also put cells in wells where they weren’t able to adhere to anything at all and looked at how they divided in suspension. We worked with Pedro Almada (Henriques Lab) to use online fixation, a technique he developed during his PhD. Using this approach, we carried out live imaging of a cell entering mitosis and then, with an automated microfluidic exchange system, we fixed and immunostained the cells at the microscope. We were then able to carry out immunofluorescence to identify adhesion proteins on fixed cells which we also had live information for. Finally, a visiting scientist in the lab, Marina Uroz, placed cells on gels with varying levels of stiffness and used traction force microscopy as well to look at how forces may play a role during mitosis.
What were the most challenging and fun parts of the project?
The most frustrating part of the project was trying to image enough cells going through mitosis. Cells don’t go into mitosis very often; furthermore, we were using migratory cells, so it was difficult to catch cells that progressed through mitosis before they wandered out of view. However, this could be resolved by just spending more time imaging. The most challenging part was figuring out how to quantify things that were quite qualitative, like cell shape. Eventually we just had to look at enough cells to be able to consistently categorise them.
I really enjoyed working with Pedro for the online fixation technique. It was really cool to be able to set up just one experiment and get information about live and fixed cells that confirmed something we had hypothesised. We were able to see that fixed cells leave integrin puncta in the same places as live cells leave zyxin puncta. You can actually exactly overlay the two forms of adhesion. I also really liked making micro-patterns and carrying out those experiments; again, the experiments yielded very nice proof of one of our hypotheses.
This paper mostly seems to be looking at fundamental cell biology, but does it have translational aspects?
The majority of the paper looks at RPE1 cells, which are a normal, non-transformed human cell line. In addition, we worked with Helen Matthews and followed on from some of her earlier work to carry out parallel experiments in human cancer cell lines, e.g. HeLa cells. We found there are differences between non-transformed and transformed cells. It seems that the cancer cell types are able to divide without adhering to the substrate, as long as they can build a contractile ring. This suggests that cancer cells are less dependent on their environment than non-transformed cells for division, which makes sense in contexts such as metastasis. However, this is very preliminary work which needs to be explored in more detail.
After your PhD, you joined the Making lab at the Crick Institute as a lab research scientist. You used microfabrication in some experiments in this paper. Did that spark your interest in microfabrication?
Absolutely. I found using microfabrication in experiments really interesting and I also got to work with Ravi Desai, the head of the Making Lab, during my PhD. Through chatting to him and the close interactions between the Baum Lab and the Making Lab, I realised very early on that this was something I really wanted to get involved in after my PhD.
Can you tell us about the work you’re currently doing in the Making Lab and what you hope to do in the future?
I’ve settled in my new job quite well, but I’m still learning a lot. My job is both training DIY users to use the equipment and ensuring the day to day running of the Making Lab goes smoothly. I’m also going to be manufacturing the parts used in projects we’ll be doing with collaborators. The work is really varied and it’s nice to be involved in lots of different projects. At the moment, I’m not missing working with cells too much and really enjoying making new tools.
Image caption: Microscopy images illustrating how daughter cells can use adhesion to migrate away from each other to complete division
Written by Gemma Wilson
 Close
Close