We work with several different mouse models of neuromuscular disease (e.g. CMT and ALS) to determine the mechanisms underlying neurodegeneration. To do this, we combine a range of standard methods (e.g., western blotting, immunohistochemistry, quantitative real-time PCR) with more specialised approaches to assess motor and sensory nerve pathologies both in vitro and in vivo:
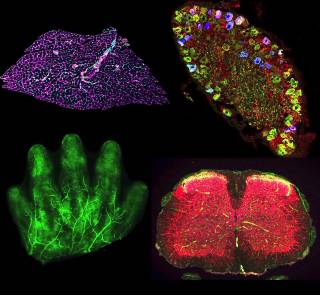
- Anatomical Dissections
Intricate dissections of the peripheral nervous system for comparative anatomical assessments. These allow us to better understand disease pathogenesis and the impact of therapeutic intervention.
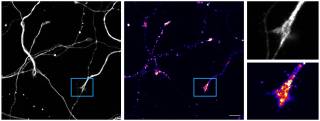
- Primary Neuron Cultures
Primary culturing of motor and sensory neurons for live imaging. This allows us to track the in vitro dynamics of diverse axonal cargoes, such as signalling endosomes, mitochondria and lysosomes. We do this in mass culture, as well as in specially designed microfluidics that allow physical and fluidic separation of cell bodies from axons and growth cones.
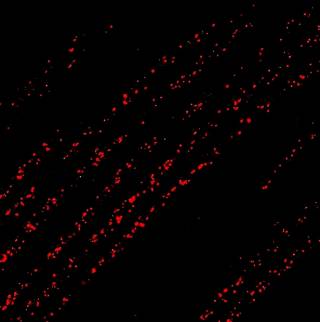
- In vivo Imaging
Live imaging of the intact mouse nervous system in vivo. We have pioneered real-time imaging of peripheral nerves in live mice, allowing us to assess the dynamics of varied cargoes (e.g., signalling endosomes and mitochondria) both in axons and at the neuromuscular junction within muscles.
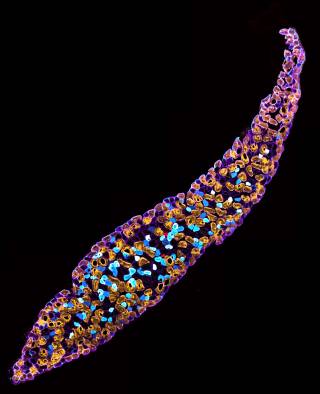
In addition to this, we are beginning to develop novel, pre-clinical adeno-associated virus (AAV) therapies for neuromuscular diseases, as well as exploring the differentiation and live imaging of induced pluripotent stem cell-derived neurons.
The development of novel techniques is critical to the advancement of our understanding of the peripheral nervous system, and we are continually adapting and improving our methodologies to aid this pursuit.
 Close
Close

