Our laboratory focuses on three major research themes that crosscut mitochondrial biology and translational science.
Establishing the global prevalence and genetic architecture of mitochondrial diseases

Understanding the role of cardiolipin in mitochondrial health and disease
Phospholipids are the major component of all cell membranes. Their amphiphilic properties enable formation of lipid bilayers that compartmentalise cells, thereby segregating metabolic pathways within highly specialised organelles. Cardiolipin is a non-bilayer phospholipid present exclusively in mitochondrial membranes that has many important roles necessary for normal mitochondrial function. Mammalian cardiolipin biosynthesis is a complex process that involves the endoplasmic reticulum and mitochondria, but it is ultimately synthesised from phosphatidic acid within the inner mitochondrial membrane. The intrinsic functional relationship between the endoplasmic reticulum and mitochondria has recently extended to mitochondrial division and mtDNA replication, which occurs at ER-mitochondria contact sites. We are studying the role of cardiolipin in these aspects of mitochondrial function using cell biology and imaging techniques, harnessing patient-derived induced pluripotent stem cell models, while attempting to identify new human disease-causing genes involved in phospholipid and cardiolipin metabolism.Generating effective therapies for mitochondrial diseases
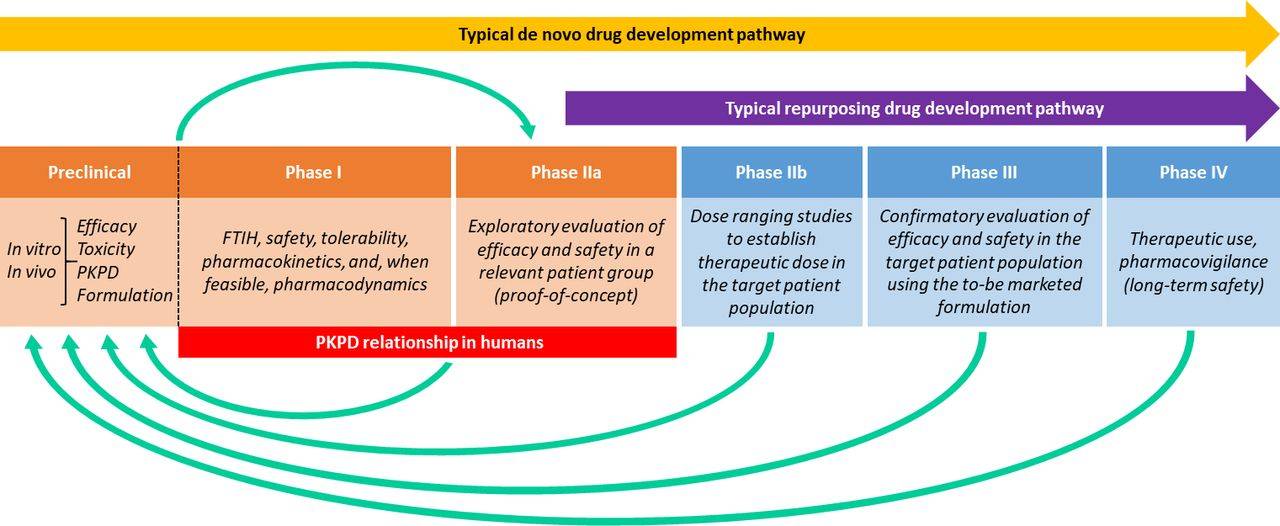
Drug repurposing strategies explore the pharmacologic action of a known drug for an indication different from licensed. However, possible improved bioavailability of repurposed compounds through changes to their dose or route of administration is often overlooked before commencing efficacy trials. Numerous vitamins, cofactors and food supplements have significant repurposing potential in mitochondrial diseases but their target tissue engagement can be poor. One such example is nicotinamide riboside (NR), a Vitamin B3 derivative and NAD+ precursor, which has proven benefits in mouse models of mitochondrial disease via enhanced mitochondrial biogenesis. We are exploring a breadth of formulation technologies to design effective delivery systems for NR, and other NAD+ precursors, to maximize tissue penetration, harnessing myogenic differentiation of induced pluripotent stem cells derived from fibroblasts of patients with genetically confirmed mitochondrial disease to evaluate the efficacy of lead formulations in preclinical and proof-of-concept studies.
 Close
Close

