Tocilizumab - a new treatment for severe juvenile idiopathic arthritis in children

12 December 2014
UCL research has led to a new treatment for patients with severe juvenile idiopathic arthritis. The novel use of tocilizumab to treat this condition has transformed patients' long-term outcome: it halts structural damage preserves joint function and can restore a normal rate of growth, reducing disability and dependence in adulthood.
Juvenile idiopathic arthritis (JIA) is the most common rheumatological inflammatory disease of children, with around 15,000 affected in the UK alone. Its most severe form, systemic onset (sJIA), accounts for around 10% of all JIA patients and can cause joint pain, damage, blindness and disability, as well as long-term sequelae (poor growth, low bone density). sJIA is also associated with potentially fatal complications such as macrophage activation syndrome.
Treatment options may be limited by potentially serious unwanted side effects. Steroid use, for example, which may be necessary to treat life-threatening aspects of sJIA, can cause its own problems, including growth delay. However, research at UCL has supported the development of the monoclonal antibody tocilizumab as a new treatment for JIA. The drug has since transformed the long-term outcome of patients. It halts structural damage preserves joint function and can restore a normal rate of growth, all of which factors help to reduce disability and dependence on health and social care throughout adulthood.
This important development has its origins in early translational work at UCL on Interleukin (IL)-6, an immune protein that causes inflammation and is produced in response to infection. During the 1990s a UCL research team led by Professor Patricia Woo (UCL Division of Infection & Immunity) was the first to demonstrate that patients with sJIA had specific genetic variations in the genes that control production of IL-6 and that these variations were associated with elevated levels of IL-6 in the blood. That finding provided the team with justification for further exploration of IL-6 as a treatment target.
By 1999, UCL was one of only two UK centres running a study into the use of tocilizumab in adult patients with rheumatoid arthritis (RA) - a separate but related condition - and the first outside Japan to treat adult patients with RA with this drug. Published in 2002, this work demonstrated the dose range with beneficial effects on joint inflammation, showing that inhibition of IL-6 significantly improved the signs and symptoms of RA and normalised the acute-phase reactants.
In 2005, Professor Woo led the first clinical, early study outside of Japan to investigate escalating doses of the drug for use in sJIA. In turn, this led to the TENDER trial, a larger, multi-centre phase III study to investigate the safety and efficacy of tocilizumab in sJIA, the results of which were published in 2012. This, the largest ever randomised, placebo-controlled study in this relatively rare disease group, was conducted over five years in 43 participating centres around Europe.
The TENDER trial demonstrated unequivocally the efficacy of tocilizumab in sJIA and led, in 2013, to it being licensed for this use by the US Food & Drug Administration and by the European Medicines Agency. In the UK, the National Institute for Health and Clinical Excellence had already recommended its use for sJIA in a technology appraisal in 2011, and tocilizumab has also been endorsed by the British Society of Paediatric and Adolescent Rheumatology. It has since been adopted as treatment for sJIA around the world.
Tocilizumab is now the standard of care in children with systemic onset JIA, offering an alternative to treatment when methotrexate fails to work. The prevalence of JIA and proportion of those with sJIA refractory to methotrexate suggests that many hundreds of young patients are likely already to have benefitted from this.
Images
- Researchers at the Centre for Adolescent Rheumatology. Courtesy Centre for Adolescent Rheumatology
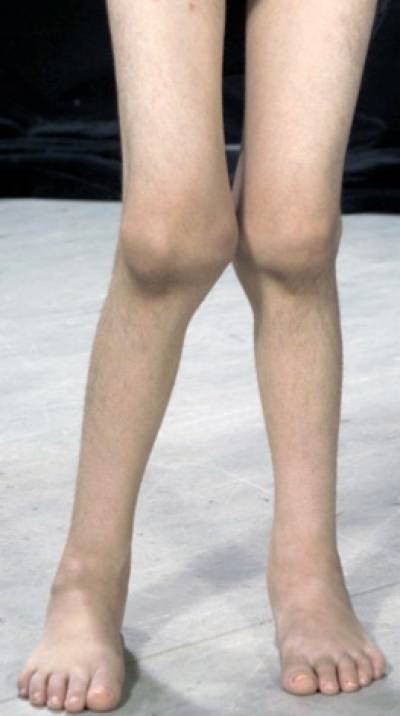
- A child's legs affected by juvenile idiopathic arthritis. Courtesy Centre for Adolescent Rheumatology
 Close
Close

