New dental treatments help to reduce development of antibiotic resistance
16 December 2014
UCL research has developed novel treatments for periodontitis, caries and other infectious diseases. These are quick, simple, and help to reduce antibiotic use. Two systems are now commercially available: Periowave, for treating periodontitis, has been used to treat an estimated 313,000 patients so far. MRSAid is used in hospitals to eradicate MRSA from the nostrils, thereby reducing the chance of post-surgical infection. Further research into new applications of the technology is ongoing.
Antibiotic resistance is a serious and growing public health concern. As pathogens develop resistance to commonly used antibiotics, our ability to treat infectious diseases is diminishing. For more than 20 years, UCL researchers have been developing a novel technology - light-activated antimicrobial agents (LAAAs) - which offer an alternative to antibiotics. LAAAs are drugs that have no antimicrobial activity in the dark but can be activated by light of an appropriate wavelength.
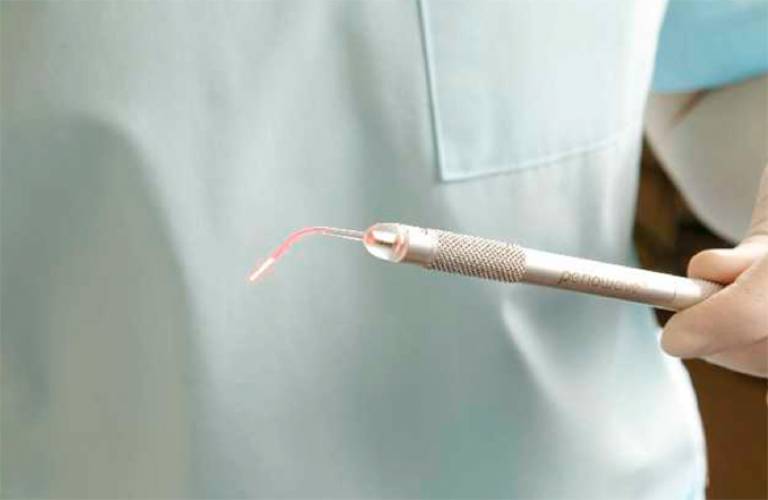
The first practical application of the research was a new treatment (Periowave) for periodontitis (inflammation of the gums and destruction of tooth-supporting tissues) which is the most common chronic infectious disease of humans. It was developed for commercial use through a collaborative research programme with Ondine Biomedical Inc.
The system consists of a hand-held light-emitting device and a syringe containing the LAAA. The dentist applies a solution of the LAAA to the disease site, then irradiates it with light from the athermal laser for 60 seconds. The treatment takes only a few minutes overall and is painless and stress-free. It means that a course of antibiotics is not necessary, reducing the risk of adverse side effects and concerns about patient compliance. Importantly, by providing an alternative to antibiotics, Periowave is helping to reduce the development of antibiotic resistance thus maintaining the effectiveness of existing antibiotics for the treatment of other infectious diseases.
Periowave is currently available in Canada, Mexico, South East Asia and a number of European countries. It has been granted CE marking and FDA approval is being sought. To date an estimated 92,000 treatment kits have been sold and 313,000 patients treated.
My first Periowave treatment was just great. It was completely painless, and it was interesting to hear scientifically what was going on. There was absolutely no discomfort. If somebody gave me a choice between Periowave and an antibiotic, I would take Periowave in a flash. No antibiotics! - Patient feedback

This novel antimicrobial approach has also been adapted for use in hospitals to eradicate MRSA from the nostrils, thereby reducing the chance of post-surgical infection. The new system (MRSAid) has been approved for use and sale in Canada and is pending approval in the European Union. Vancouver General Hospital recently demonstrated that the system reduced post-surgical infection by almost 40% and saved the hospital $1.3m in related costs.
Further applications of LAAAs being investigated are: preventing catheter-associated infections (by fabricating catheters from LAAA-containing polymers); reducing the microbial load on hospital surfaces (by coating surfaces with LAAA-containing polymers); and preventing wound infections (using dressings containing LAAAs).
This research has been supported by the MRC, BBSRC, EPSRC, the Charles Wolfson Charitable Trust and Ondine Biomedical Inc.
All images courtesy of Ondine Biomedical Inc.
 Close
Close

