Find out about Professor Han Stauss' research on T cell therapy of cancer.
We use gene transfer and gene editing technologies to engineer the specificity and function of therapeutic T cells.

Immunotherapy with T cells has shown efficacy in cancer patients who failed conventional therapy. Adoptively transferred T cells can develop effector function leading to tumour destruction. In contrast to cancer treatment with monoclonal antibodies, which typically requires repeated treatment to maintain systemic antibody levels, T cell therapy is a one off treatment that can results in long-term protection.
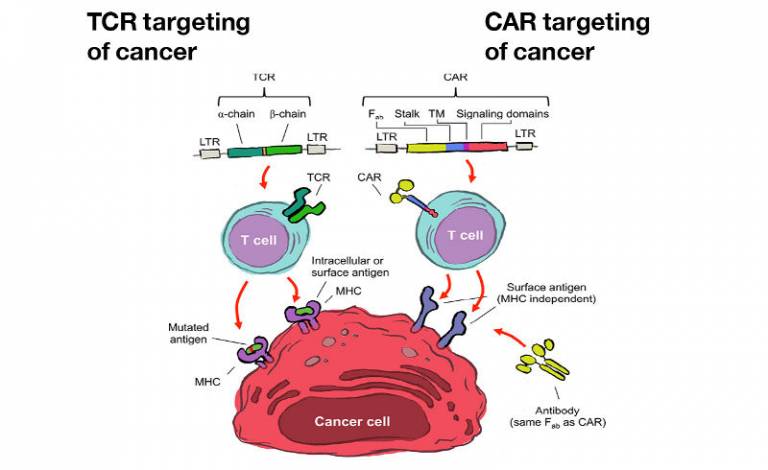
Genetic engineering has been used successfully to rapidly and reliably generate T cells of defined antigen specificity. Retro- and lentiviral vectors can efficiently transfer the genes encoding T cell receptors (TCR) or chimeric antigen receptors (CAR) into T cells. The TCR allows T cells to recognise any intracellular and cell surface protein, while CAR can only recognise proteins expressed on the surface of cancer cells.
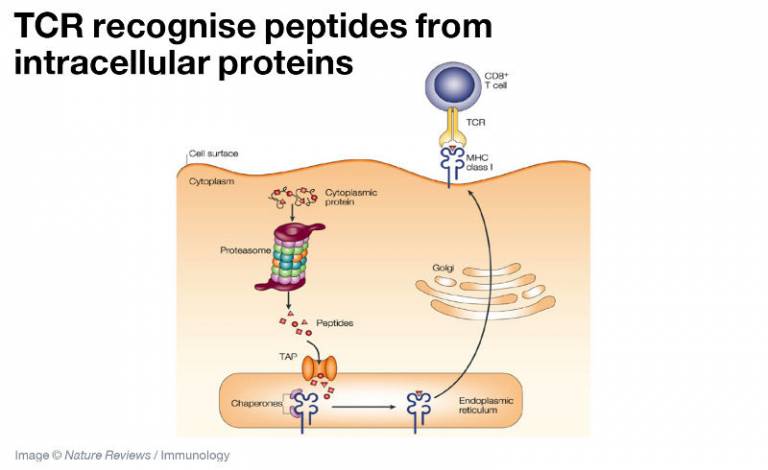
Intracellular proteins, including cytosolic signalling molecules and nuclear transcription factors, are broken down into peptide fragments by the proteasome complex, transported into the endoplasmic reticulum by the TAP transporter and then loaded into the peptide binding groove of MHC class I molecules. The MHC/ peptide complex migrates to the cell surface where it is recognised by the TCR.
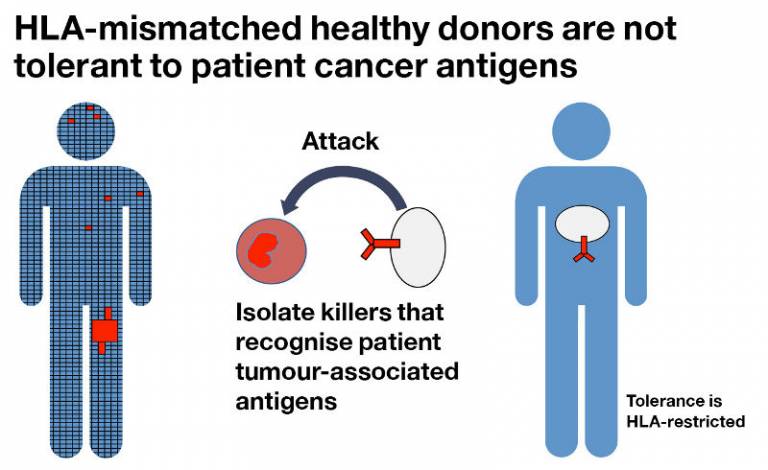
We have developed a platform technology to isolate T cells that can recognise tumour-associated antigens that may induce tolerance in the autologous T cell repertoire. T cells from MHC mismatched healthy individuals are not tolerant to the tumour associated antigens presented by patient MHC molecules, allowing the isolation of high avidity T cells specific for these antigens.
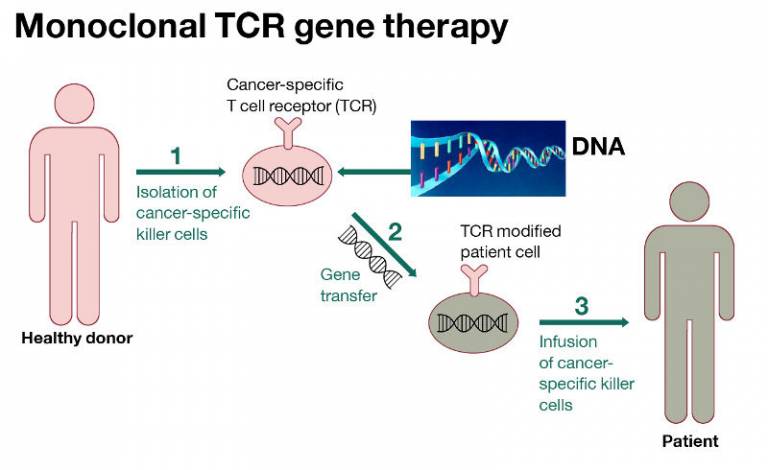
Tumour-reactive TCR can be isolated from the repertoire of healthy individuals. Retro- and lentiviral vectors are used to transfer the TCR genes into the T cells isolated from the peripheral blood of patients. The patient T cells acquire tumour-specificity that allows them to attack cancer.
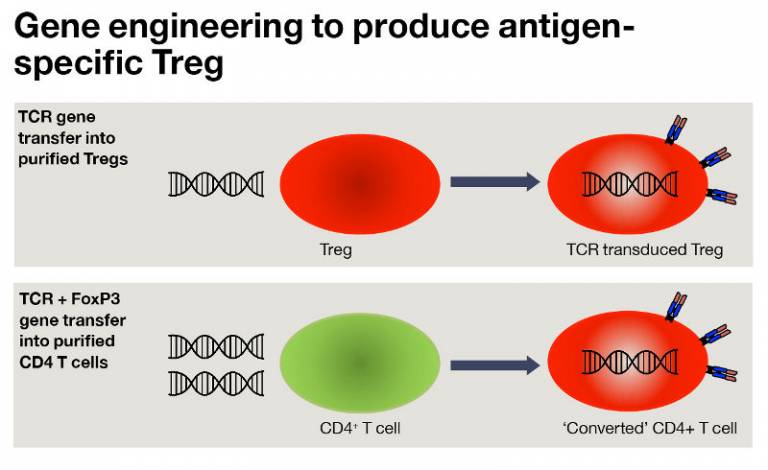
We have employed gene transfer to produce antigen-specific regulatory T cells (Treg) using two approaches. TCR gene transfer into purified Treg, or TCR plus FoxP3 gene transfer into CD4 T cells. Both approaches produce Treg that suppress in the presence of the TCR recognised antigen, but not in its absence. This provides a therapeutic strategy to suppress unwanted immune responses in vivo without the need for systemic immune suppression.
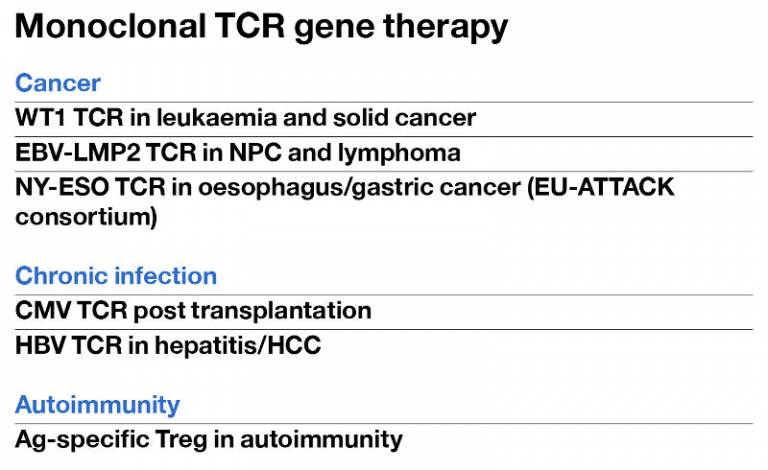
This is a summary of the current and future plans to test TCR gene therapy strategies in clinical trials.
 Close
Close

