Department of Structural and Molecular Biology
Division of Biosciences
University College London
Darwin Building - Gower Street
WC1E 6BT London, UK

Ruth Pritchard
Former PhD Student (Wellcome Trust)
Email: ruth.pritchard[at]ucl.ac.uk
New post doc at University of Sussex, UK.
Research
Background:
I have a long-standing interest in method development, particularly the integration of diverse experimental and theoretical approaches in order to address the intricacies and subtleties of biological systems. In 2008-2009 I was fortunate enough to work with Dr Brad Amos at the MRC-LMB on the development of a high-resolution, low magnification 'mesolens'. Later I also had the opportunity to work on the development of a high-speed neuronal imaging system using surface plasmon resonance techniques with Dr Noah Russell in iBIOS, Nottingham.
I graduate with a BSci(hons) in Biochemistry from the University of Edinburgh completing my honours research project with Prof. Manfred Auer working on the development of a fluorescence-based binding assay for medium-throughput drug discovery. My interest in interdisciplinary science and its importance to so many of the most intriguing problems in biological science was what lead me to choose the ISMB's Structural, Chemical and Computational Biology PhD program.
Project in the Hansen Lab:
A significant proportion of biological systems are highly dynamic in nature with motions observable across a wide range of time-scales. In many cases, inherent conformational flexibility of both backbone and side-chains has been shown to be critical to function. Despite this, traditional approaches in structural biology are strongly targeted towards the backbone conformation of static entities. The methods that are available for probing rare states and flexible systems often yield very limited data. As such, there is a very real need both for novel experimental methods and sophisticated theoretical models to integrate data drawn from a range of diverse sources.
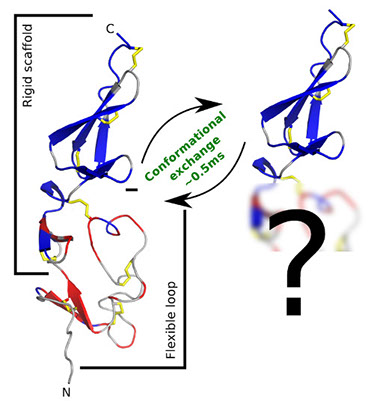
My PhD focuses on the development of NMR spectroscopic methods reporting specifically on the conformational sampling of side-chains and how to combine these, and other, experimental parameters into all-atom molecular dynamics simulations. As well as working with a number of well-characterised model systems, I am using these methods to understand the behaviour of the critical haemostatic protein von Willebrand Factor. The TIL'-E' domains of this protein form an interaction with Factor VIII which is critical for proper blood clotting. These domains contain both a well folded region and a flexible loop which has been shown to be present in at least two states in solution. I am working to characterise the motions in these domains and identify the conformation of the minor state by drawing together existing and new experimental parameters into enhanced sampling molecular dynamics simulations.
Figure 1: Flexibility in the TIL'-E' domains of von Willebrand Factor
These domains form the primary binding surface for the essential haemostatic interaction with coagulation Factor VIII. Parts of the backbone are static on a millisecond time-scale (blue) but the loop region is dynamic on this time-scale (red) and has been found to be in conformational exchange with a minor state occupying 3.5% of the solution population. Grey indicates regions where it was not possible to obtain data. I am using a range of sophisticated experimental and theoretical techniques to identify the structure of the minor state and determine how these dynamics relate to function.