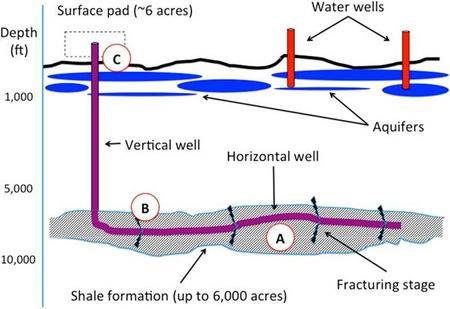
Hydraulic fracturing or fracking is the extraction of natural gas, mainly methane, from shale formations. These rocks have high porosity (6 – 12 %) but low permeability, which means large volumes of gas can be trapped within a formation. To extract these gas reserves the shale is fractured using fluids to enhance pre-existing or create new fractures, allowing the permeability of the shale to increase and gas to flow from the shale.
Hydraulic fracturing has been implemented since the late 1940s in the United States. While only a modest amount of gas was recovered the technology has been refined to create improved methods of drilling and extractions. New research has also lead to previously discarded sites being explored and deemed to be economically viable.
Recoverable worldwide shale gas reserves have been estimated to be set at about 6600 trillion cubic feet (tcf) which increases the global natural gas resources by 40%. In the UK the shale gas reserves have been estimated at 20 tcf.
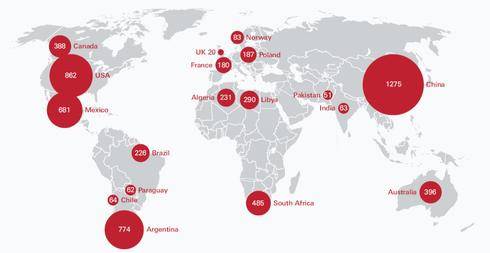
Figure 1. Estimates of the technically recoverable shale gas resources in trillion cubic feet based on 48 major shale formations in 32 countries. Source. Shale gas extraction in the UK.
How Shale Gas is Extracted
In 2012 the Royal Society and the Royal Academy of Engineers recommended that the recovery of shale using hydrofractruing techniques should go ahead but also detailed a list of safety protocols for companies undertaking hydraulic fracturing operations. In the US, a recent study by the Environmental Protection Agency examined the potential impacts of hydraulic fracturing.
BG group awarded full sponsorship for the four year project ‘A new strategy for predicting free gas in shale gas using carbon, nitrogen and noble gases’ based in UCL that commenced on 1st January 2013.
Dr. Sudeshna Basu, Senior Research Associate at UCL, is responsible for the science of the project, which will be administered jointly with Dr. Adrian Jones at UCL. The project will also draw on a number of other researchers namely Dr. Alex Verchovsky (Open University, UK) and Prof. J. Lambaise (Chulalongkorn University, Bangkok). This is an industry-academic joint venture between UCL and BG, with Mr. Jonathan McQuilken representing BG Group as part of a new BG Shale Gas Hub for research. Some of the gas analyses will be conducted in the UCL noble gas geochronology laboratory, using a recently fabricated sample crusher installed by PDRA Dr. James Schwanethal, and Prof. Pieter Vermeesch.
PROJECT DESCRIPTION
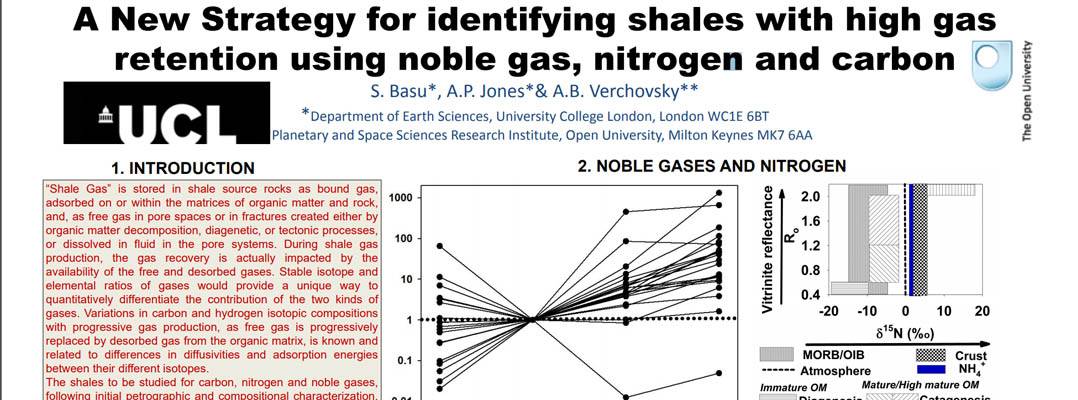
Improvements in the extraction efficiency and understanding of the geochemistry of shale gas reserves are important global topics in the progress to utilise this source of unconventional natural gas. This requires better estimates of the volumes of extractable gas from free pore space and from structurally bound gas in minerals. Both values are required for predicting and optimizing ultimate gas recovery, but there is very little data. The project focuses on shale geochemistry and shale characterisation using new approaches and ideas including a combination of carbon, nitrogen and noble gases to estimate the proportions of free vs. bound gas present, and will enable early identification of shale source rocks with high gas retention.
This research has been presented at the 2013 Goldschmidt meeting in Florence, Italy (Poster and abstract below).
Jabraan Ahmed (PhD Student) Institute for Sustainable Resources
Research Outline. The main aim of this research is to reduce the risk of hydraulic fracturing to communities by improving the understanding of factor controlling shale deposition, its mechanical properties and gas generation. The project will address these issues using shale deposits from the Bowland Basin, by characterising these deposits in terms of porosity, permeability and geochemistry. Further investigation using high resolution imaging on micro to nano scale will enable the distribution and orientation of minerals and kerogen.
Supervisors. Prof. Juergen Thurow, Prof. Neal Skipper, Prof. Philip Meredith and Dr Joanna Faure-Walker
Mike Chandler (PhD Student) ExxonMobil URC / UCL Impact
Research Outline. Hydraulic fracturing is important in obtaining gas from shale formations, but accurate predictions of the directions and extent of fractures are required to perform operations safely and improve the efficiency of gas extraction. Fracture toughness, the resistance of the rock to dynamic crack propagation, is considered to be a significant factor determining the progress of artificially created fractures. This project uses rock physics experiments to investigate the toughness of shale with varying temperature and pressure conditions. Corresponding numerical modelling of these processes has also been undertaken to compare with experimental results.
Supervisors. Prof. Philip Meredith, Dr Nicolas Brantut and Dr Brian Crawford (Exxonmobil)
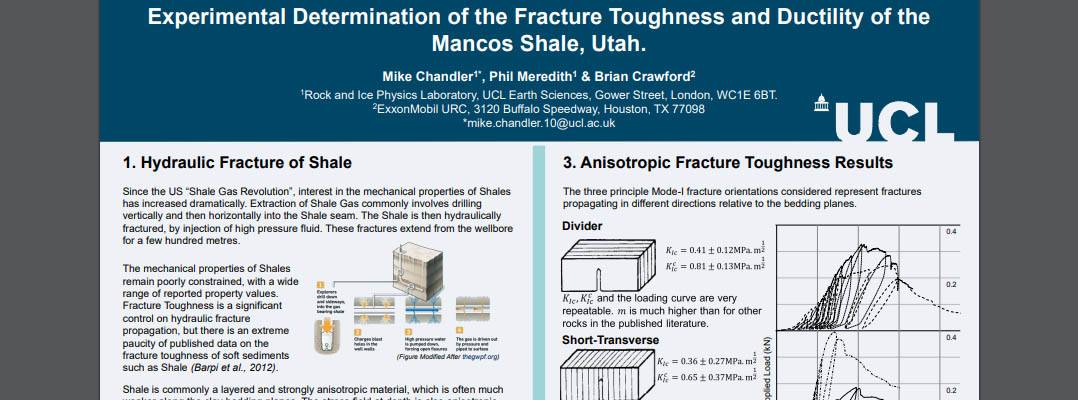
M. Chandler, P.Meredith and B. Crawford, "Experimental Determination of the Fracture Toughness and Brittleness of the Mancos Shale, Utah.", 75th EAGE Conference & Exhibition. London: UK (June 2012)
Professor Alberto Striolo
Tuan Ho
Department of Chemical Engineering
Within the chemical engineering department at UCL ongoing research is exploring physical chemical properties of water, salt, and hydrocarbons confined within narrow pores such as those present in subsurface shale formations. The fundamental results from our studies could help develop hydraulic fracturing in a more sustainable way – e.g., using less water, extracting more hydrocarbons, and releasing fewer contaminants.
The questions we address were identified by scientists from industry, academia, and government, gathered in 2012 at a workshop organized by Striolo and supported by the US National Science Foundation.
- Example 1: Does Methane Mix with Shale Water?
During hydraulic fracturing high-pressure water is used to crack shale rocks. During this process, water comes in contact with methane, and other hydrocarbons. Methane and water do not mix at ambient conditions. But what happens in the shale rocks?
We seek to understand whether methane mixes with the water present in the small pores present within a shale formation. We employ massive computer modeling for answering this question. Our preliminary results suggest that methane does mix with shale water!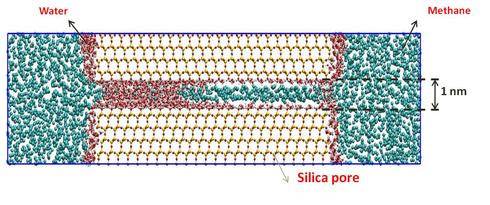
- Example 2: How Much Gas Is Present In Shales?
Shale rocks are fractured by hydraulic processes to enhance the permeability of gas and oil through the formation. The goal is to be able to extract the hydrocarbon stored in the formations. But, no two shale formations are alike: can we predict how much hydrocarbon is present in one formation before we fracture it?
We employ computer modeling to predict the amount of hydrocarbons, for example propane, a component of natural gas, in simple representations of pores. We can then explore the effect of rock type (e.g., quartz vs. kerogen), pore size, formation temperature and pressure, on the amount of gas stored.
- Example 3: Methods for Water Reclamation
During the process of fracturing, water comes in contact with the shale formation. Once the procedure is completed, part of the water returns to the surface containing substances that cannot be released to the environment (e.g., large concentrations of salt). Can we design economical technologies to efficiently extract the undesired substances from water?
We are focusing on membrane processes to enhance water desalination. In a recent contribution, graphene-based membranes were explored.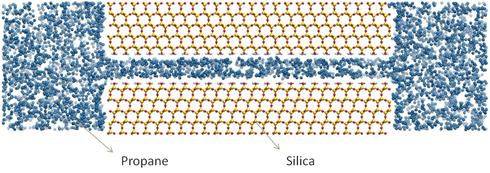
Note that this research could be applied to desalinate seawater and satisfy the world’s increasing thirst for drinking water.
- References
D. Argyris, A. Phan, P.D. Ashby, A. Striolo, Hydration Structure at the α-Al2O3 (0001) Surface: Insights from Experimental AFM Force Spectroscopy Data and Atomistic Molecular Simulations, J. Phys. Chem. C 117 (2013) 10433-10444.
D. Konatham, J. Yu, T.A. Ho, and A. Striolo, Simulation insights for graphene-based water desalination membranes, Langmuir 29 (2013), 11884. Cover art.
X.-C. Luu, J. Yu, A. Striolo, Nanoparticles Adsorbed at the Water/Oil Interface: Coverage Effects on Structure and Diffusion, Langmuir 29 (2013) 7221-7228.
A. Yethiraj, A. Striolo, Fracking: What Can Physical Chemistry Offer?, invited commentary, J. Phys. Chem. Lett. 4 (2013) 687-690
T.A. Ho, D. Argyris, D.R. Cole, and A. Striolo, Aqueous NaCl and CsCl Solutions Confined in Crystalline Slit-Shaped Silica Nanopores of Varying Degree of Hydroxylation, Langmuir 28 (2012) 1256–1266.
A. Phan, T.A. Ho, D.R. Cole, and A. Striolo,Molecular Structure and Dynamics in Thin Water Films at Metal Oxide Surfaces: Magnesium, Aluminum, and Silicon Oxide Surfaces, The Journal of Physical Chemistry C 116 (2012) 15962-15973. Cover art.
A. Striolo, Surface Adsorption of Colloidal Brushes at Good Solvent Conditions, J. Chem. Phys. 137 (2012) 104703/1-9.
D. Argyris, T.A. Ho, D.R. Cole, and A. Striolo, Molecular Dynamics Studies of Interfacial Water at the Alumina Surface, Journal of Physical Chemistry C 115 (2011) 2038-2046.
H. Fan, A. Striolo, Nanoparticles Effects on Water-Oil Interfacial Tension, Phys. Rev. E 86 (2012) 051610/1-11.
T.A. Ho, D.V. Papavassiliou, L.L. Lee, A. Striolo, Water Can Slip on a Hydrophilic Surface, Proceedings of the National Academy of Sciences of the United States of America 108 (2011) 16170-16175.
A. Striolo, Surface Adsorption of Colloidal Brushes at Good Solvent Conditions, J. Chem. Phys. 137 (2012) 104703/1-9.
Shale gas is commonly known as an unconventional energy source, from which many have the potential to replace fossil fuels as one of the major source of energy. research at University College London also focuses on other forms of unconventional energy.
Coal Bed Methane (CBM)
Paul Sutherland (PhD Student)
Dr William Burgess.
Sponsored by BP
What is Coal Bed Methane?
Coal bed methane is natural gas contained in most coal formations, where coal is both the source and reservoir rock, unlike conventional gas reservoirs. These reservoirs of gas are typically found between 150 and 1000 m depth. Methane is mainly stored within the molecular structure of the coal, but can also be stored within fractures or 'cleats' within the coal as a gas or dissolved within water. The methane exists at hydrostatic pressure and by reducing the pressure, methane desorbs from the coal surface and flows into fractures. The pressure can be reduced by partial dewatering of the coal bed methane strata.
The main environmental issues associated with CBM are: 1) large volumes of produced water requiring disposal; 2) depletion of shallow groundwater resources; and 3) long-term groundwater recovery and restoration of the hydrologic balance.
Research at UCL
Current research is focusing on the principal hydrological variables controlling the degree and location of influences of CBM dewatering on shallow aquifer conditions. Using idealised basin structures, the anisotropy of the CBM basins is being analysed by conceptual and numerical modelling. This is being used to determine characteristic groundwater age and groundwater head profiles as a guide for targeting environmental impacts at an early stage at operational sites. The overall aim is to improve understanding of the environmental impacts of CBM development so as to guide monitoring priorities, by addressing the following questions:
1. To what extent are CBM formations influenced by actively circulating groundwater (e.g. what is the relationship between groundwater flow and the occurrence of biogenic methane?).
2. What are the implications of CBM basin structure for the environmental impact of CBM development?
 Close
Close

