Map work exercises, geology, supplementary material
Geology around UCL
- The Quadrangle

Poorly repaired limestone steps

Horizontal bedding revealed by raised fossils

Large raised fossil in Portland limestone

Weathered York Stone flags showing laminations
- The Japanese Roof Garden
Content placeholder

Black gabbro monument on granite plinth
Close up of granite basal plinthGrey granite on roof garden wall

Yellow brick wall

Yellow brick building with slate roof
- The Cloisters
Content placeholder
Marble statue of Flaxman, South Cloisters


Marble base of Santarelli's Lost Innocence statue
- The Henry Morely Building

Oolitic limestone window sill
Damaged portion of window sill revealing oolites in the limestone

York Stone flags outside the Henry Morley building
Close up, showing weathering of the York Stone flags, and revealing their fine laminations
Building Materials
Select a material from the menu on the right for further study.
- Clays and Clay Minerals
Clay is the common name for a number of fine-grained, earthy materials that become plastic when wet. Chemically, clays are hydrous aluminium silicates, usually containing minor amounts of impurities such as potassium, sodium, calcium, magnesium, or iron.
One of the commonest processes of clay formation is the chemical decomposition of feldspar. Clay consists of sheets of interconnected silicates combined with a second sheet-like grouping of metallic atoms, oxygen, and hydroxyl, forming a two-layer mineral such as kaolinite. Sometimes the latter sheet like structure is found sandwiched between two silica sheets, forming a three-layer mineral such as vermiculite. In the lithification process, compacted clay layers can be transformed into shale. Under the intense heat and pressure that may develop in the layers, the shale can be metamorphosed into slate.
Properties of clay minerals include plasticity, shrinkage under firing and air drying, fineness of grain, colour after firing, hardness, cohesion, and capacity of the surface to take decoration. On the basis of such qualities, clays are variously divided into classes or groups.
Individual clay particles are always smaller than 0.004 mm. Clays often form colloidal suspensions when immersed in water, but the clay particles flocculate (clump) and settle quickly in saline water. Clays are easily moulded into a form that they retain when dry, and they become hard and lose their plasticity when subjected to heat.
Clays are divided into two classes:
Residual clay – found in the place of origin
Transported clay, also known as sedimentary clay, removed from the place of origin by an agent of erosion and deposited in a new and possibly distant position.
Residual clays are most commonly formed by surface weathering, which gives rise to clay in three ways:Chemical decomposition of rocks, such as granite, containing silica and aluminia
Solution of rocks, such as limestone, containing clayey impurities, which, being insoluble, are deposited as clay
Disintegration and solution of shale.
Clay rocks can be identified by their very fine grain size of < 0.002 mm, and have different properties depending on which particular clay minerals they contain.There are three main groups of clay minerals, each with its own particular properties:
Kaolinite
Illite
Montmorillonite
Clay rocks may contain a mixture of these minerals, so they have very variable properties, giving rise to a number of different uses. The most abundant use of clay is in brick making.Granite is made up of quartz, mica and feldspar. As quartz is resistant to chemical weathering, it may be removed only as mineral grains of quartz. Feldspars and micas are susceptible to chemical weathering and break down to form clay minerals.
Some of the original elements contained in the micas and feldspars are carried away in solution as ions (Na+, Ca+, and K+), and so the clays formed are relatively enriched in aluminium and silicon.
The main group of clay minerals are kaolinite, illite and montmorillonite. The layers in kaolinite are held together by fairly weak bonds, whereas there is strong bonding in illite and montmorillonite because of the presence of positively charged metal ions; potassium in the case of illite, and calcium and sodium in the case of montmorillonite.
Generally, potassium feldspar breaks down to form kaolinite; micas weather to give illite, and ferromagnesian minerals break down to form montmorillonite.
Clay uses
Clay has been used since the very beginnings of civilisation, for making cooking pots, bricks, porcelain, and also drainage pipes.Both brick clays and other clays are used for other purposes, such as the manufacture of clay pipes, and for floor and wall tiles. Fireclays are used for more refractory purposes such as heat-resistant tiles or bricks. Ball clays are used for ceramics. China clay, predominantly kaolinite, is used in ceramics, as a filer and in drug manufacture. Expanded clays are used as a lightweight aggregate in the manufacture of expanded clay blocks used for insulation. However, the major use of clay, after brick manufacture, is in the manufacture of cement.Highly absorbent, bentonite is much used in foundry work for facing the moulds and preparing the moulding sands for casting metals. The less absorbent bentonites are used chiefly in the oil industry, e.g., as filtering and deodorizing agents in the refining of petroleum and, mixed with other materials, as drilling muds to protect the cutting bit while drilling. Other uses are in the making of fillers, sizings, and dressings in construction, in clarifying water and wine, in purifying sewage, and in the paper, ceramics, plastics, and rubber industries.
Clays and subsidence
One important aspect of clays as far as construction goes, is the possibility of subsidence where a building has been constructed on clay. This was a big problem in the hot dry summers of the late 1970s and 1980s in southern England. Most clay rocks contain a high proportion of natural water (up to 40%), filling all the minute pore spaces between the mineral grains, but because these spaces are so small, water cannot pass through the rock, and it is impermeable. Clay topsoils dry out in a long hot summer, shrinking as they lose water, and develop cracks in the surface layer. If the ground is also being dried out at depth by tree roots – that is, the underlying clay is being dewatered – the clay can lose enough water in a long, hot summer for it to shrink enough to cause part of the building to settle or subside, especially if the house foundations are shallow. Cracks may then develop in the building, requiring expensive remedial work to underpin the foundations. In some cases it is possible to stop the damage by cutting down the tree, and so allow the clay below the house to rehydrate. However, if the tree has been there a long time, rehydration of the clay as it reabsorbs water and swells up can itself cause further ground movements and damage to the house, a process known as heave. The moral of this tale is don’t plant large thirsty trees near your house!Clay sources in the UK
UK brick production mainly uses clays from the Cretaceous Wealden formations, the Jurassic Oxford Clay, the Triassic and the Carboniferous. China Clay is produced in vast quantities from deposits in SW England above the Cornish granites.- Brick
Brick is a ceramic structural material that, in modern times, is made by pressing clay into blocks and firing them to the requisite hardness in a kiln. Bricks in their most primitive form were not fired but were hardened by being dried in the sun. Sun-dried bricks were utilized for many centuries and are used even today in regions with the proper climate. Examples from approximately 5,000 years ago have been discovered in the Tigris-Euphrates basin, and the ancient races occupying this region may have been the first users of brick. In Babylonia there was a lack of both timber and stone, and the thick clay deposited by the overflowing rivers was the only material adaptable to building. The Persians and the Assyrians used sun-dried blocks of clay for walls of great thickness, facing them with a protective coating of fired bricks. The Egyptians and the Greeks used bricks only to a limited extent, as they had access to plentiful supplies of stone and marble. The Romans manufactured fired bricks in enormous quantities and gave them an important role as a basic structural material in buildings throughout the Roman Empire. Bricks played an important part in early Christian architecture until the decline of the empire. Whereas the Romans had usually concealed their brickwork beneath a decorative facing of stone or marble, the Byzantines devised a technique for exposing the bricks and giving them a full decorative expression. This technique influenced the Romanesque style and brought especially good results in Lombardy and in Germany, where bricks came to be arranged in immensely varied patterns. Since the Middle Ages, brickwork has been in constant use everywhere, adapting itself to every sort of construction and to every change of architectural style. At the beginning of the 19th cent. mechanical brick-making processes began to be patented and by the latter half of the century had almost entirely replaced the ancient hand-fashioning methods. Contemporary American building bricks are rectangular blocks with the standard dimensions of about 5.7 x 9.5 x 20.3 cm. Good bricks are resistant to atmospheric action and high temperatures and are more durable than stone. Where heat resistance is especially important, fire bricks are used; these are made of special refractory clays called fire clays and are fired at very high temperatures.
Brick making
Bricks have been around for a long time – in fact they were introduced by the Romans into this country, and by the Middle Ages, they began to be used in churches and other important buildings. After a lot of wooden houses were burnt down in the Great Fire of London in 1666, many houses were rebuilt in brick. They became even more important during the Industrial Revolution and it is only in the last 50 years that use has declined because of the increasing importance of concrete and other cement-based materials in building factories, bridges and houses.
Most clays will make reasonable bricks. Once clay has been dug out, it is ground and mixed with enough water to allow it to be shaped to form “green” bricks. These are then dried slowly, and then fired in a kiln at somewhere between 1000 and 1200°C.
At these high temperatures, the clay is “metamorphosed”. All the water is driven off, and new anhydrous minerals are formed, which are stable at high temperatures. Kaolinite breaks down to form aluminosilicates such as the mineral mullite and quartz. This is a kind of dehydration reaction. Once they have been fired, the bricks contain an interlocking network of long, thin mullite crystals, quartz, and some supercooled liquid (glass) which make the brick hard and strong. Where does the colour come from? Most natural clays contain iron minerals, either oxides or hydroxides. Iron can exist as ferrous iron in the reduced state (iron II), in which case it forms dark grey oxides and hydroxides, and this causes some sediments to be dark in colour. After firing, however, this iron may be oxidised to the iron III state, or ferric state, which forms the red-brown iron oxide haematite.
Other minor metal constituents in natural clay include Ca+, Na+ and K+, and these can cause melting by forming silicate liquids which speed up the alteration of the clay minerals. This silicate liquid cools to give a glassy coat to the particles, and helps to make the brick hard. The amount of liquid has to be kept low to stop the bricks from going a funny shape or fusing together. On the other hand, if high strength bricks are needed, e.g. for building railway bridges, a relatively high proportion of the brick is allowed to melt under reducing conditions (giving a blue-grey brick), so that the pores are sealed by glass, giving an impermeable, much stronger brick. This type of brick used to be used in the nineteenth century for damp-proof courses in houses. Old bricks fired at low temperatures may remain porous and permeable, and let damp come in through the walls.
Brick production and uses of bricks
The output of bricks has changed enormously between 1948 and today. For example, the total output of bricks in 1990 was about 4 billion, whereas in 1965, the output was 8 billion. The main use for bricks is house-building, which has dropped dramatically since the 1960s. This is probably because of the substitution of other materials (e.g. cement-based blocks), and because building regulations dictate higher standards of thermal insulation than before, so lightweight blocks are made from a variety of other materials. These have a high porosity, which give better insulation, and they are also therefore lighter, so they can be manufactured into much bigger volumes than a standard brick. Walls made of these bigger blocks are cheaper and quicker to build than ones made with conventional bricks.
Past and future use of bricks
As well as being used for house building, the Victorians used enormous quantities of bricks for other purposes, many of which were engineering bricks, e.g. warehouses, factories, mill chimneys, and railway viaducts. These bricks were often made from Carboniferous clays and shales, which were plentiful around the industrial centres of the British coalfields. One of the reasons for the decline in the number of bricks being used is because of substitution (prior to the 1950s, bricks were still used for load-bearing walls, but high rise flats and offices now tend to use a frame made out of steel or concrete to carry the structural load, and quite often even the walls are made out of other materials, either concrete, glass or other non load-bearing material. Where bricks are used, they usually form a layer only 1 brick thick. Whereas the Victorians used bricks for their railway viaducts, motorways built today use concrete for the motorway itself, the bridges and retaining walls. Airport runways also use vast amounts of concrete.
Another example of substitution in the use of brick-making clays is the manufacture of roof tiles. Nowadays, few houses have roofs that are made of clay tiles, or slate for that matter, because they have been replaced by cement-based tiles. Underground, the Victorian sewers were largely brick-lined, and smaller pipes were often made of earthenware. Large diameter pipes are made of concrete or cast iron, and smaller diameter pipes are made of plastic.
The biggest user of bricks remains in housing, and reserves of brick clay are not a problem in the foreseeable future.
- Limestone
Limestones belong to the group of sedimentary rocks known as chemical sediments. They are formed in a marine environment from the precipitation of calcium carbonate, the calcium having been brought into the sea via the hydrological cycle. Fossil shells are often found in limestones, and show that biological activity is very important too. Where CO2 concentration is low, calcitecan be precipitated directly without any biological help, e.g. Bath Stone, which is an oolitic rock. The spherical ooids are though to have been precipitated as concentric layers built up around particles rolling around the sea floor. Pure limestone can be made up completely of calcite, but if other material is being laid down as well, such as sand, then the limestone will be impure.
British Limestones
The three most important limestones in Britain are:
1. Carboniferous Limestone
This is a well-cemented rock of low porosity, and occurring in thick beds. As they have prominent vertical joints, they can be easily spilt into blocks for use as building stone. It crops out, for example, in the Peak District, Mendips and Yorkshire Dales. It contains most of the country’s potholes and natural cave systems. Mostly, Carboniferous limestone is either a fine calcite mud, precipitated from warm shallow seas, or a shelly limestone, formed by fragments of animals such as corals. It is a very tough rock, and is commonly used as roadstone, but can also be used for cement making because it is often quite pure, and as a source of calcium carbonate for the chemical industry.
2. Jurassic Limestones
These occur in the Cotswolds, and are a pale brown colour, much softer and more porous than the Carboniferous Limestone. These are often oolitic, and occur in beds a few metres thick. These are important as building stones, especially in the Cotswolds. In Bath and at Portland, they are sufficiently well-cemented to be used as freestones (e.g. Portico steps). They are not usually strong enough to be used as roadstone, but are sometimes pure enough to be used for cement-making.
Limestone from Isle of Portland
3. Cretaceous Limestones
These are the various chalks that dominate much of SE England, such as the North and South Downs. Chalk is a white, fine-grained porous rock, formed from the remains of planktonic organisms, and made up of very fine plates of calcite. You can only make out bedding where there are rows of flints. Chalk is generally too soft to be used for building, but is used a lot in cement-making.
USES OF LIMESTONE
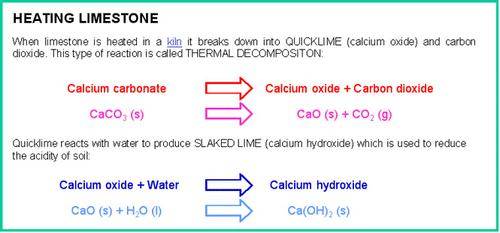
Limestone is a commonly occurring rock which can be used not only as a building material but also for making many other useful substances including lime, cement and glass.
If limestone is powdered it can be used to neutralise the acidity in lakes caused by acid rain and to neutralise acidic soils.
Cement is produced by roasting powdered limestone with powdered clay in a rotary kiln. When cement is mixed with water, sand and crushed rock, a slow chemical reaction produces a hard, stone-like building material called concrete.
Glass is made by heating a mixture of limestone, sand and soda (sodium carbonate).
- Cement and Concrete
How is cement made?
The basic characteristic of cement is that after it has been mixed with water, it will set hard as rock, and will bind together any rock or mineral fragments mixed into it. Mortar is made from a mixture of sand and cement, and bonds together bricks in a wall. Most cement is mixed with both sand and aggregate to make concrete.It seems that the Romans knew about cement, but the technique was lost until Smeaton built the Eddystone Lighthouse in 1756, using a mixture of fired ground limestone and clay. Cement was patented by John Aspdin in 1824 as Portland cement because he though it looked rather like limestone from Portland, which was used a lot in buildings. This type of cement is used a lot today, and is still known as OPC (ordinary Portland cement).
What are the ingredients?
Calcite (CaCO3) from limestone
Silica (SiO2)
Alumina (Al2O3)
+ minor amounts of iron.
All of these ingredients can be assembled by mixing limestone and shale. After grinding them together, they are fired in a kiln to about 1400°C. Water is given off first, showing that the shale is decomposing, and then CO2, when the limestone starts to decompose. The other materials react to produce cement clinker.The four most important anhydrous components of Portland Cement are, in decreasing order of abundance: tricalcium silicate, dicalcium silicate, tricalcium aluminate, and tetracalcium aluminoferrite.
The cement clinker is obviously anhydrous and decarbonated. It is ground up into a powder to form ordinary OPC. When this is mixed with water, new hydrated minerals form. These grow as long crystals, locking the cement and any inert particles into a hard mass.
These new hydrated minerals form slowly, and the cement needs to be kept moist while this is occurring. Portland cement starts to harden a few hours after mixing, because the hydrated tricalcium aluminate grows rapidly. Cement doesn’t start to become strong until some days later when the hydrated tricalcium silicate forms. It reaches 70% of full strength after about a month, and doesn’t reach full strength until several years later because the dicalcium silicate hydrates so slowly. This reaction is non-reversible.
Geology and raw materials for making cement
Occasionally, sediments already have roughly the correct proportions of minerals present to make cement. Cementstones are impure limestones that contain quartz and clay minerals and some iron. Sometimes they are interbedded thin limestones and shales that will together give the right composition for making cement. Usually, a cement works will be built where limestone and clay crop out next to each other. The raw material is then ground up in the right proportions for making the cement. It is common in southern England for a cement works quarry to have chalk at one end, and clay at the other. The mixing becomes more tricky where the composition of the limestone or shale is variable.Case Study
There is an interesting example of land restoration at a limestone quarry in Dunbar, where land is restored behind the excavation as the working face advances. There is a very expensive bridge conveyor that moves the waste from one end of the quarry to the back, but the quarry life is about 50 years, which is a long time, and it allows the quarry waste to fill up the old workings continuously, so that only a narrow strip of the quarry is exposed at any one time.Uses of cement
Mortar for bonding bricks is made up of 1 part by volume of cement powder with 3 to 6 parts of sand, and the minimum of water to make the mix workable. Cement and sand can also be used to produce a thin skin or render, to protect the outside of the buildings. The most important use of cement is in making concrete, where it is used to stick together a mixture of sand and rock fragments, i.e. aggregate.
 Close
Close


