Types of Clinical Trials
There are different types of clinical trials which are designed according to the questions they are looking to answer.
Phases of a Clinical Trial
Clinical trials are split into stages to test a new treatment, these are called phases. Phases allow researchers to answer different questions about a new treatment depending on where we are in the discovery process as each phase has a specific objective. Having a clinical trial in phases ensures researchers recruit the correct number of participants to answer the questions and allows researchers to sequentially increase the number of participants involved as we look at different objectives.
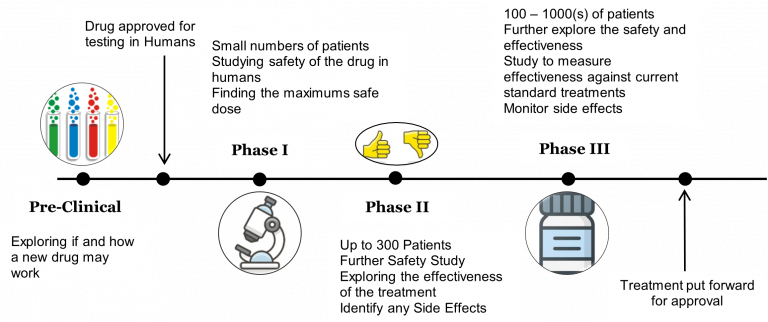
Phase I | These trials involve a small number of people (~20-50 people) and look at what happens to the treatment in the body and if there are any side effects. The trial also looks to find the best dose for the treatment. |
Phase II | These trials involve a medium number of people (~over 100 people) and looks at how well a treatment works. |
Phase III | These trials involve a large number of people (~100s or 1000s of people) and it aims to compare the new treatment against the standard treatment option to check if the new treatment is better and/or has fewer side effects. |
Phase IV | These trials occur after the treatment has been put forward for approval. They involve 100s or 1000s of people depending on the investigation and look at finding out more about the long-term benefits and side effects of the new treatment. |
Some trials have an earlier stage called Phase 0 so researchers can check a drug behaves as they observed in laboratory testing.
What are Early Phase Clinical Trias?
Early phase trials may look at whether a drug is safe and the side effects it causes. Later phase trials aim to investigate whether a new treatment is better than the existing treatments.
Phase I
Phase I trials are usually small trials, recruiting only a few patients with the aim of finding out the safety of the treatment and the side effects. It will try answer questions like how well does the body get rid of the drug, how effective is it and does the treatment help to shrink the cancer.
These studies will aim to find the best dose for the treatment, in what are called Dose Escalation Studies.
Phase I studies will have stricter inclusion criteria so will recruit at a slower pace. Patients will have lots of blood tests to ensure the researchers understand the treatment, how the treatment works and how the treatment affects the body.
Phase II
Phase II studies will recruit more people, up to 100 or so. Phase II will continue to assess the safety and side effects of the treatment, and also answer questions on which cancer the treatment works on, effectiveness on the cancer and also get more information about the best dose.
Aims of an Early Phase Trial
Safety Assess the safety of experimental drugs |
Side Effects Evaluate any possible side effects |
Dose Determine a safe doe range. |
Reaction To see how the body reacts to the drug, how it is absorbed, distributed, and eliminated from the body, and the effects that it has on the body. |
Taking Part in a Clinical Trial
If your clinician/oncologist believes you would benefit from taking part in a particular clinical trial, they will refer you to the Principal Investigator conducting the trial at UCLH.
If you would like to take part in a clinical trial, you should speak to your GP or clinician. Please Note only your clinician can make this referral for you.
If you take part in a clinical trial, or are considered for a clinical trial, you will first be given information about the study.
 Close
Close

