We are investigating the very first steps in the developmental (and perhaps evolutionary) history of inner ear sensory organs...
We know that the sensory organs are derived from 'prosensory' patches, which are specified in the early otic vesicle. The prosensory domains express the transcription factor Sox2, as well as the Notch ligand Jagged1, which are both essential for the maintenance of the prosensory fate. But do these prosensory domains form separately (at some distance from one another), or do they share a common embryonic origin?
We found that in the chicken and the mouse, several of the sensory organs are produced by segregation from a common ‘pan-sensory’ domain. In the anterior sensory-competent region of the otic vesicle, the pan-sensory domain gives rise to two prosensory patches, giving rise to the cristae, and later on the utricle... so there is after all a close (spatial, at least) relationship between these different organs before they become fully separate from one another.
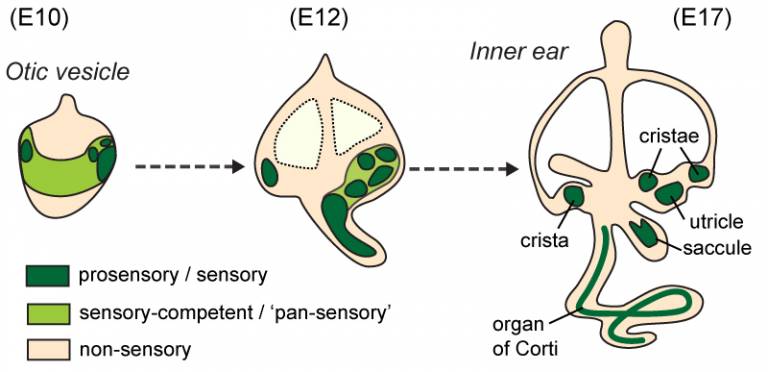
A schematic representation of sensory organ formation in the mouse inner ear, with approximate stages in days of embryonic development (E).
This segregation process is somehow comparable to the geological drift that led to the separation of the continents, although sensory organ ‘tectonics’ obviously proceeds at a much faster pace! This mechanism was suggested some time ago by the anatomists (eg Retzius in the 1880’s) who studied the inner ears of fish and amphibians… but we can now explore sensory organ segregation with much better tools and genetic models. For example, we have shown that this segregation is at least partly dependent on changes in cell character: some of the early sensory-competent cells switch off Jagged1/Notch activity, Sox2 expression and form non-sensory 'boundary' territories between adjacent sensory organs. We have also found that one transcription factor expressed in non-sensory 'boundary' territories, Lmx1a, is one of the key signals controlling these changes in cell character: when it is absent, sensory organs fail to segregate. When it is overexpressed, new 'boundaries' between sensory organs appear to form.
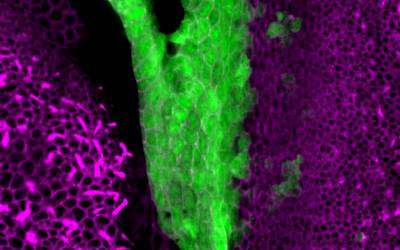
Surface view of the vestibular organs in a neonate mouse. On the left, a crista. On the right, the utricle... and in between, the Lmx1a-expressing cells (green).
So here's our new motto: "prosensory specification only makes sense in light of sensory organ segregation"!
This work could help us understanding how the inner ear has acquired new functionalities in the course of evolution. In fact, the early jawless vertebrates (resembling contemporary lampreys or hagfish) had a very rudimentary vestibular inner ear, specialized in sensing head movements. Fish, and their descendants who colonized the land, evolved new inner ear organs, enabling them to sense water and ground vibrations or hear air-borne sounds – some serious technological innovations in the evolutionary arms race between preys and predators!
Another question we have been recently working on is: why are prosensory domains forming in the ventral domain of the otocyst only?
Using genetic "tricks" in chicken embryos, we found that this is because the Wnt signalling pathway, one of our major cell-to-cell communication system, prevents otic cells located dorsally from adopting a prosensory or neuronal character.
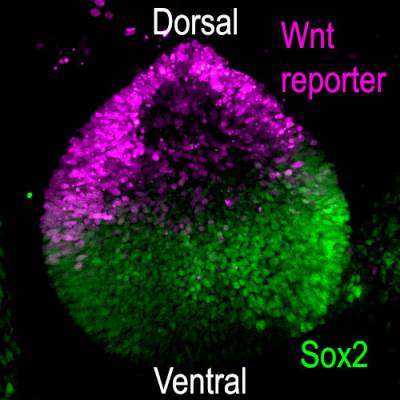
Wnt activity and Sox2 expression in the chicken otic vesicle (day3)
Wnt molecules are soluble - they diffuse away from their production site and can at a distance induce changes in gene expression in target cells. In the otic vesicle, we found that high levels of Wnt activity repress the expression of Sox2. Because Wnt factors are produced in the dorsal part of the otic vesicle, Sox2 becomes progressively restricted to the ventral half of the vesicle, where prosensory domains arise. Remarkably, when we blocked Wnt activity at the otic vesicle stage, new prosensory domains and otic neurons formed dorsally.
Could the manipulation of Wnt activity induce the formation if sensory progenitors or neurons in the adult inner ear, for example after damage? Possibly, but the adult cells of the inner ear are very different from their embryonic progenitors and it is likely that other "prosensory factors" need to be present or activated for this to work.
We hope that our research into the mechanisms of early inner ear development will help to uncover new prosensory genes and signals that could be used in the future for regenerative and stem cell therapies.
Collaborators: Vincent Plagnol (UCL Genetics Institute)
Publications:
- Żak, M. and Daudet, N. (2021). A gradient of Wnt activity positions the neurosensory domains of the inner ear. eLife 2021;10:e59540. [see BioRxiv 2020.05.04.071035 for an earlier draft]
- Daudet, N., and Żak, M. (2020). Notch Signalling: The Multitask Manager of Inner Ear Development and Regeneration. Adv. Exp. Med. Biol. 1218, 129–157. [the preprint version pdf of this chapter can be downloaded here]
- Shaping of inner ear sensory organs through antagonistic interactions between Notch signalling and Lmx1a. Journal article; 2017; eLife, 6. doi:10.7554/elife.33323
- Daudet, N., Ariza-McNaughton, L., Lewis, J. (2007). Notch signalling is needed to maintain, but not to initiate, the formation of prosensory patches in the chick inner ear. DEVELOPMENT, 134 (12), 2369-2378. doi:10.1242/dev.001842
 Close
Close

