The CADET Project: The Children’s Adaptive Deep brain stimulation for Epilepsy Trial.
About the CADET project
Objectives
The CADET Project is a series of consecutive clinical device trials that aim to investigate the safety, feasibility and effectiveness of thalamic DBS to treat children with Lennox-Gastaut syndrome.
The centromedian nucleus of the thalamus will be targeted bilaterally.
The Picostim DBS device
The CADET Project is using a cranially-mounted DBS device called the Picostim with software called DyNeuMo-1, manufactured by Bioinduction Ltd.
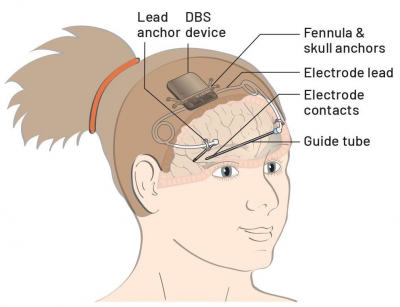
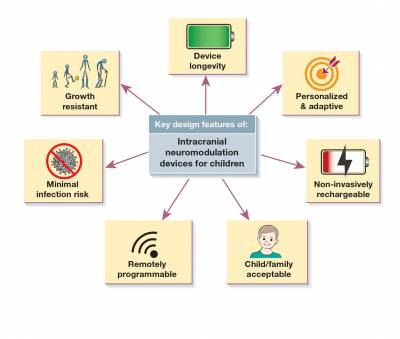
While the Picostim has not been trialled in humans with epilepsy, it has several features that are attuned to the needs of DBS for childhood epilepsy:
- Although not used within the CADET Pilot, the advanced capabilities of Picostim DyNeuMo-1 allow for personalised and adaptive stimulation regimes according to seizure patterns and circadian (wake/sleep) rhythms at an individual patient level, which are key components of LGS symptoms. These capabilities are here proposed to maximise the potential of DBS to limit seizure spread, and reduce seizure frequency and severity.
- These capabilities are made possible by the ability to non-invasively and repeatedly recharge the device. Patients or parents are able to use a handheld or headset-mounted charger that safely recharges the battery through the skin.
- The device allows for the recording of electrical activity in the implanted brain, potentially allowing stimulation parameters that are tailored to LGS and to the individual child.
- Lastly, the whole device is mounted to the skull, removing the need for (painful) tunnelling of extension leads to a battery implanted in the chest wall and potentially reducing the risk of infection.
For further reading on the design of neurostimulation devices for children, please read our commentary in the Lancet Adolescent and Child Health.
CADET Pilot
Our first clinical trial is called ‘CADET Pilot’ will investigate the safety of the Picostim DBS device with Lennox-Gastaut syndrome and the feasibility of our next and larger ‘CADET Trial’. Four children with Lennox-Gastaut syndrome will be recruited and treated at Great Ormond Street Hospital and King’s College Hospital.
CADET Pilot is funded by the Royal Academy of Engineering and sponsored by University College London.
For further details, including the eligibility criteria, please see our clinicaltrials.gov webpage.
CADET Trial
The ‘CADET Trial’ will follow the CADET Pilot and will investigate the effectiveness of DBS to reduce seizure frequency in 22 children with Lennox-Gastaut syndrome. Further details will be available following completion of recruitment to the CADET Pilot.
CADET Trial is funded by the LifeArc / GOSH Children’s Charity Translational Acceleration Programme.
 Close
Close




