EPSRC/Jeol Centre of Liquid Phase Electron Microscopy
A transmission electron microscopy (TEM) unit to image samples in liquid phase with sub-nanometer spatial resolution and sub-millisecond temporal resolution. This facility opened on July 2017 at the department of Chemistry and combines some of the newest developments on electron imaging to create a unit dedicated to liquid samples. In doing so the EPRSC/Jeol Centre of Liquid Phase Electron Microscopy aims to:
- Provide much needed capacity building across a range of applications involving soft matter, biomaterials, biological physics, synthetic biology, nanomaterials design and catalysis.
- Pioneer new imaging techniques exploiting the unique liquid nature of the samples to establish Brownian 3D tomography techniques.
The Centre for Liquid Transmission Electron Microscopy imaging (LTEM) comprise a TEM microscope equipped with an in-column omega filter, a direct detection camera, a conventional CCD camera for analytical TEM, a conventional tilt holder for grids, and a liquid holder connected with several liquid handling units to allow for inflow analysis.
Equipment
- JEOL-JEM2200 FS Microscope
The JEM-2200FS, a state-of-the art analytical electron microscope, is equipped with a 200kV field emission gun (FEG) and an in-column energy filter (Omega filter) that allows acquisition of zero-loss image, where inelastic electrons are eliminated, resulting in clear images with high contrast. Energy-filtered images are formed with electrons at low loss or core loss energy and can provide chemical maps or elemental information of a sample. Also, spectroscopy for elemental analysis and chemical analysis of specimens is available. JEM-2200FS also offers high quality imaging on STEM mode
In-column energy filter (Omega filter)
The in-column energy filter enables the user to obtain energy-filtered images and electron energy loss spectra. The optimally designed filter provides distortion-free filtered images.
Control system
The main components of the JEM-2200FS, such as the optical system, goniometer stage and evacuation system, are fully PC-controlled. This system stably produces high-quality data.
Imaging system
A new imaging system, consisting of four-stage intermediate lenses and two-stage projector lenses, achieves rotation-free energy-filtered TEM images and diffraction patterns over a wide range of magnifications and camera length.
Piezo-controlled goniometer
A new goniometer stage that incorporates a piezo device offers smooth operation for searching fields of view at the atomic level.
Integration to other instruments
The microscope can be fully controlled with PC. The design concept enables us to integrate EDS system and CCD cameras.
STEM detectors
At the bottom of the projector lenses, conventional scanning transmission-electron microscope (STEM) image detectors are installed. These detectors include bright-field BF detector, high-angle and upper high angle annular dark-field HAADF and U-HAADF detectors respectively. The dark-field image from the HAADF detector shows Z-contrast that depends on the average atomic number without interference effects from the electron beam. Combined use with an EDS allows 2D mapping of the element distribution in the specimen.
U- HAADF is installed at the top of the energy filter. You can insert or retract all these detectors into or from the optical axis by remote control using GUI, and observe each image on the PC monitor.

Photo of the JEOL JEM 2200 FS microscope displaying also the digitizer of the K2 at the bottom with the orange cables
- UltraScan 1000XP CCD camera for conventional and analytical energy-filtered imaging
Energy-filtered transmission electron microscopy (EFTEM) is a an imaging technique that use characteristics of the energy loss spectrum to enhance image contras. The main applications include:
- Contrast enhancement – Improves contrast in images by removing inelastically scattered electrons that produce background fog including zero-loss filtering. This feature is particularly useful for organic low contrast materials.
- Mapping – Creates elemental/chemical maps at nanometer resolution by forming images with inelastically scattered electrons
- Analytical – Records and quantifies electron energy loss spectra (and maps) to provide chemical analysis of TEM samples
The principle behind EFTEM is based on the illumination of a very thin specimen with a high energy electron beam. When most of the incoming electrons pass through the specimen, some will interact with the specimen and result in elastic or inelastic scattering. Inelastic scattering results in both a loss of energy and a change in momentum, which in the case of inner shell ionization is characteristic of the element in the sample.
- K2 IS Direct Detection Camera for low dose imaging
High performance direct detection in-situ camera from Ametek (Gatan) allows to resolve dynamic details in heating, catalysis, mechanical deformation, electrical testing, and chemical reaction experiments.
Counting and in-situ studies
- 14.2 Megapixel direct detection sensor reads out at 400 fps with each frame delivering a usable image
- Highest DQE maximizes signal-to-noise ratio
In-situ studies
- Full- or sub-area sampling at rates up to 1600 fps
- Direct data storage with >15 minutes capture time, regardless of frame rate
- Longest direct detector lifetime even when using highest dose rates
- Streamlines large dataset management for both novice and expert users
- Video buffer allows you to capture only the video you want; with post-event triggering and LookBack
- Usable individual frames
Counting studies
Retains full capabilities of K2 Summit modes (counting and super-resolution) for beam-sensitive materials for both materials science (zeolite, metal-organic framework (MOF)) and biological applications (cryo-tomography, SPA) structural characterization.

- Stream Liquid Holder
Liquid Phase Electron Microscopy (LTEM) offers a step-change in our ability to study matter in its virgin state on the nano and micron scale, removing the artifacts induced by drying or cryogenic treatments. LTEM imaging find applications in a myriad of research fields from electrochemical reactions, nanocrystals growth, dynamic processes, and tomographic reconstructions of particles in liquid to name a few.
The Stream in-situ liquid holder from DENSsolutions allows the capability to visualize and capture dynamics in liquid samples. The liquid samples are placed in a micro-fabricated fluidic chamber – called the Nano-Cell. The Nano-Cell ensures the specimen is fully hydrated and the liquid can be controlled in either a static or flowing condition. The bottom chip contains both inlet and outlet channels, surrounded by spacers that define the microfluidic channel. Liquid enters the chip from the inlet, passes over the central area where the electron transparent observing window is placed and exits via the outlet.
This system allows control of liquid motion (static or continuous), flow rate and liquid thickness by reducing the level of window bulging. The liquid holder at the EPSRC/JEOL Centre for LTEM has a heating unit that allows heating up liquid samples up to 80C while in-situ imaging.
Applications:
- Nanoparticle Science - Nanoparticle synthesis, mechanisms of nucleation & growth, shape and aggregation, assembly kinetics, corrosion studies, batteries, electro catalysts
- Cell & Biological systems - Extra cellular vesicles, DNA and chromosomes, cells, virus
- Biochemistry & Nanobiotechnology - protein-protein interactions, 3D structure reconstruction via tomographic techniques, drug delivery systems, nanoparticle toxicity, dynamic processes
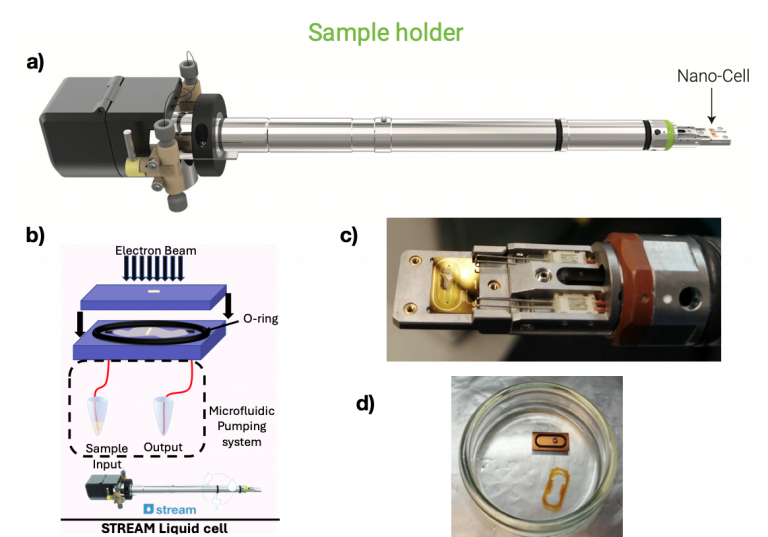
a) Stream liquid holder from DENSsolutions at the centre for LTEM at UCL. Taken from https://denssolutions.com
b) Schematic diagram of liquid cell displaying top and bottom chip, observing window, O-ring, and microfluidic pumping system, provided by Gabriel Ing; Photograhs showing in detail
c) the tip of the holder with the bottom chip, still wet after LTEM imaging, and
d) the top chip with the O-ring still wet after LTEM imaging.
- Images
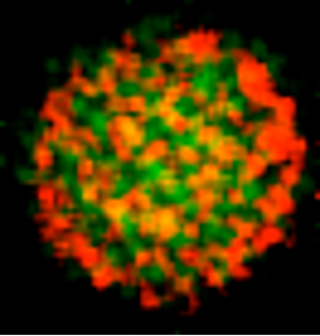
- Elemental map on a 50nm diameter polymersome from a phosphorus-rich block-copolymer obtained by Energy-Filtered Transmission Electron Microscopy (EFTEM). Green and red signals correspond to oxygen and phosphorus regions respectively.
 Scanning Transmission Electron Microscopy (STEM) image using the UDF detector of a conductive polymer on a block copolymer micelle template in absence of staining.
Scanning Transmission Electron Microscopy (STEM) image using the UDF detector of a conductive polymer on a block copolymer micelle template in absence of staining.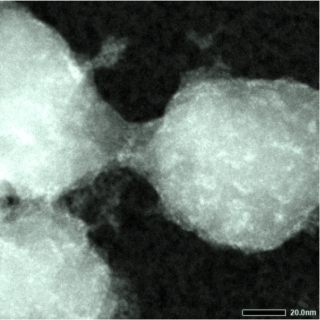
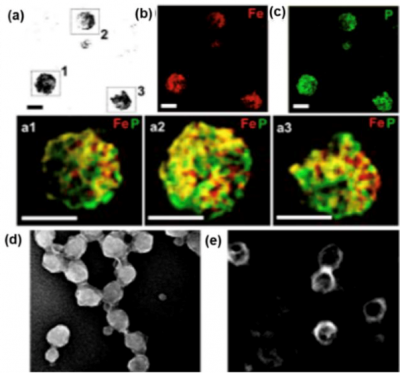
TEM micrographs of Fe3+-complexed conductive polymer on vesicles showing the a) zero-loss imaged nanostructures; energy filtered TEM micrographs displaying b) Fe elemental map (red signal), c) P elemental map (green signal) and a combination of the two elements Fe and P superimposed to zero-loss image a1,a2,a3. Scanning transmission electron microscopy (STEM) micrographs displayingFe3+-complexed d) micellar and e) vesicular nanoparticles in dark field highlighting the iron content in the structures core due to its high contrast. Scale bar = 100 nm.Reproduced from from Soft Matter 16 (19), 4569-45732, 2020
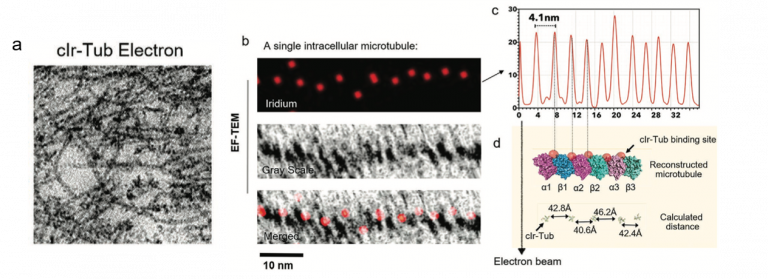
a) Conventional TEM imaging of cell microtubules stainsd with clr-Tub compound b) Conventional TEM image, iridium elemental mapping and merged images of a single intracellular microtubule. c) The spacing between the Ir signals in the microtubule is periodic with an average distance of 4.1 ± 0.3 nm. d) Comparison of this measurement with the distance between the Ir-Tub complex (red spheres) tubulin binding sites as shown on the microtubule models. Reproduced from Adv. Mat 32 (39), 2070296, 2020
Liquid TEM
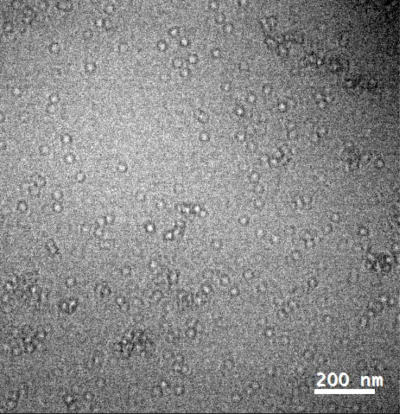
Single frame of apoferritin (label free) in PBS solution at 2.5mg/ml imaged in liquid TEM. K2 in linear dose fractionation mode. Total number of frames 1200; Frame rate: 10 frames/second; 0.1s exposure for each single frame. Pixel size 0.37nm x 0.37 nm e-dose angstrom 20 e/A^2/s. 10Kx mag. Binned 6 times to increase signal to noise. Reproduced from bioRxiv doi: https://doi.org/10.1101/2021.01.21.427613
Publications & Events
- Selected Publications
Publications where the reported imaging was performed at the EPSRC/JEOL Centre for Liquid Electron Microscopy at UCL.
L. Ruiz-Pérez, C. De Pace, G. Marchello, and G. Battaglia* “Organic and biological materials in liquid phase TEM” Imaging and Microscopy, https://analyticalscience.wiley.com/do/10.1002/was.0004000111.
C. De Pace, S. Acosta-Gutierrez, G. Ing ,G. Marchello, S. Pilotto, F. Werner, N. Wilkinson, F. L. Gervasio, L. Ruiz-Pérez,* and G. Battaglia* “Imaging protein conformational space in liquid water”. BioXiv: https://www.biorxiv.org/content/10.1101/2021.04.30.442083v1
X. Tian, C. De Pace, L. Ruiz‐Pérez, B. Chen, R. Su, M. Zhang, R. Zhang and G. Battaglia* “Live‐Cell Imaging: A Cyclometalated Iridium (III) Complex as a Microtubule Probe for Correlative Super‐Resolution Fluorescence and Electron Microscopy” Advanced Materials 32 (39), 2070296, 2020 (Front cover article)
G. Marchello, C. De Pace, A. Duro‐Castano, G. Battaglia*, and L. Ruiz‐Pérez* “End‐to‐end image analysis pipeline for liquid‐phase electron microscopy” Journal of Microscopy, 2020, doi:10.1111/jmi.12889
L. Ruiz-Pérez, L. Rizzello, J. Wang, N. Li, G, Battaglia*, and Y Pei* “Polypyrrole and polyaniline nanocomposites with high photothermal conversion efficiency” Soft Matter 16 (19), 4569-45732, 2020
Y. Zhu, A. Poma, L. Rizzello, V. M Gouveia, L. Ruiz‐Pérez, Giuseppe Battaglia,* and Charlotte K Williams* “Metabolically Active, Fully Hydrolysable Polymersomes” Angewandte Chemie International Edition 58 (14), 4581-4586, 2019
X.Tian,* S, Hussain, C. de Pace, L. Ruiz-Pérez, and G. Battaglia* “Zn(II) Complexes for Bioimaging and Correlated Applications” Chemistry - An Asian Journal , 14 (4), 509-526, 2019
- Liquid Phase Electron Microsopy Workshop November 2019
- EPSRC/Jeol Centre of Liquid Phase Clectron Microscopy Opening Symposium July 2017

 Close
Close

