Introduction
Nuclear imaging via emission tomography is highly sensitive and enables studies of a wide range of physiological and pathological processes in vivo. Nuclear imaging is based on the detection of gamma rays that are emitted by radionuclides injected into the organism. Images depict the spatial distribution of the radionuclides in the body according to their pharmacokinetic behaviour. Two nuclear imaging techniques exist, and can be distinguished due to the use of different types of radionuclides; single photon emission computed tomography (SPECT), and positron emission tomography (PET).
With the advance of preclinical imaging technology it is now possible to image the distribution of picomolar amounts of radiopharmaceuticals with a high spatial resolution of <1mm within minutes to tens of minutes. Moreover, combining SPECT and PET with anatomical imaging, such as X-ray CT or MRI, enables the precise localisation of these radiopharmaceuticals. This sensitive technology enables further understanding of the mechanisms of disease and the development of novel therapeutic strategies.
Central to emission tomography are the radiopharmaceuticals (also known as imaging probes or tracers) which are designed to localize in specific organs/tissues or enter into physiological function and highlight metabolic information. Radiopharmaceuticals generally consist of a radionuclide attached to a chemical moiety or pharmaceutical specific for a biological target, such as an endogenous receptor.
Such radiopharmaceuticals are used clinically for diagnosis and therapy of a wide range of conditions and diseases. Some commonly used radiopharmaceuticals are:18F-FDG, a radiolabeled glucose analogue used in PET imaging to study glucose metabolism; 99mTc-HMPAO (exametazime) used in SPECT imaging for the detection of regional cerebral blood flow; 99mTc-Sestamibi used in cardiac imaging to study blood perfusion; and 131I-tositumomab used in radioimmunotherapy to treat follicular lymphoma.
We are actively pursuing the development of novel tracers to non-invasively probe biological process in animal models of disease.
Facility resources
- Imaging systems: The preclinical nuclear imaging facility at CABI houses a state-of-the-art dual modality NanoSPECT/CT system along with a nanoScan PET/CT scanner. Both offer sub millimetre spatial resolution and up to 35µm X-ray CT resolution.
- Radionuclide's and radiopharmaceuticals: Together with the Centre for Radiopharmaceutical Chemistry, lead by Dr. Erik Arstad, the facility benefits from some of the most advanced chemical and analytical equipment as well as access to a wide range of PET and SPECT isotopes and radiopharmaceuticals, both established and experimental.
- Analytical resources: to complement in vivo imaging studies, the facility offers extensive equipment and resources enabling automated gamma counting for radiotracer/pharmaceutical biodistribution evaluation, cryostat sectioning of isolated organs, fluorescent phosphorimaging, and radio-HPLC/TLC to examine blood and serum metabolites.
- Multidisciplinary, multimodality: amidst a team of multidisciplinary scientists, nuclear imaging studies are enriched by information obtained from parallel imaging modalities. Each imaging modality has inherent strengths and weaknesses, and this is why at CABI we are developing multimodal methods whereby animals can be imaged sequentially on multiple platforms (Optical, MR, ultrasound, photoacoustic) so that maximum information can be gathered to answer a biological question.
- Cell culture: Our in vivo imaging systems are supported by designated cell culture facilities in CABI for in vitro radiotracer analysis.
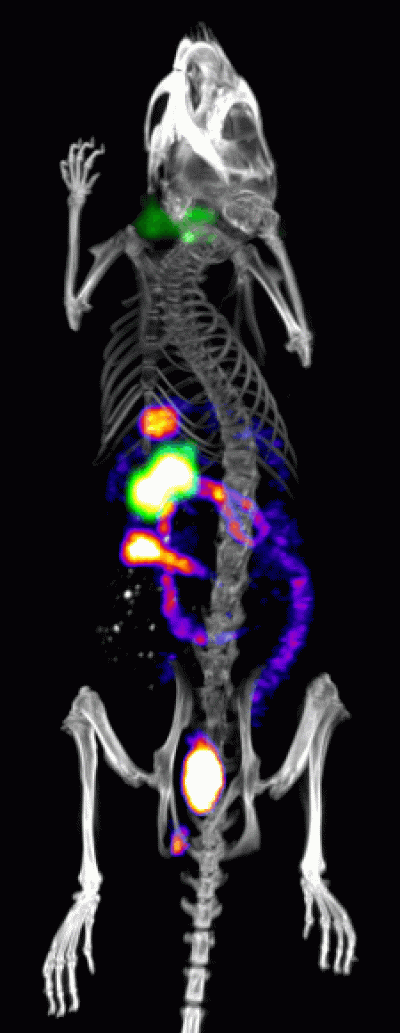
Imaging tumour resistance to therapy
See the collaborations page for information on access to this system
 Close
Close