Research theme 4
Theme Lead: Professor Adrian Thrasher
Manufacturing cells for therapeutic application
We developed automation and standardisation of the complex stem- and T-cell processing procedures to aid initial process development and eventual scale out of manufacturing. This aim aligned with the strengths and strategic imperatives of NHSBT to be a major partner in the future development of early phase studies in the area, as well as providing the skills and resources for later phase investigations, and ultimately routine delivery of therapies that become the standard of care.
Plain English research summary: Editing DNA to develop universal CAR-T cell therapies
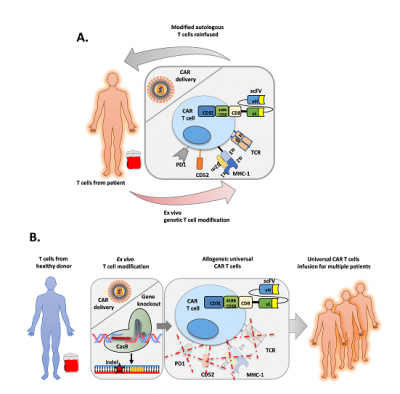
Image credit: Roland Preece, PhD
1 December 2020 - T cell immunotherapies have to be made with the patient’s own white blood cells to prevent the treatment from turning on the body of the patient and causing graft versus host disease (donated cells attacking the recipient’s body). The need to produce these therapies for each individual patient limits the wide-spread availability of this therapy as 1) it requires a centre with the expertise to make them and 2) the relatively long time to produce the therapy (~2 weeks) is not be suitable for all patients.
Read the full summary:
 Close
Close


