Immunotherapy study shows key mechanisms for targeting tumours
16 April 2018
Scientists are a step closer to understanding why some cancer patients respond well to immunotherapies while others may not, according to a new study led by UCL researchers.
In collaboration with the Royal Marsden Hospital and the Francis Crick Institute, the results of this study, published in Cancer Cell, describe key mechanisms that underlie the activity of clinically validated anti-CTLA-antibodies. The research team’s discoveries could help predict which patients are more likely to respond to anti-CTLA-4 therapies and inform the development of antibodies with enhanced activity.
CTLA-4 is a protein present on T-cells that acts as a type of ‘off switch’ to keep the immune system in check. However, this off switch can prevent the body from attacking cancer cells. Anti-CTLA-4 antibodies are designed to stop the CTLA-4 protein working, which in turn can help boost the the body’s immune response against cancer.
“Use of anti-CTLA-4 drugs such as ipilimumab offers the potential for durable remissions in patients with advanced melanoma, kidney and lung cancer, however such responses are limited to a modest fraction of treated patients. Side effects related to therapy can be significant, so we’re interested in understanding how the antibody works and identifying ways of improving its activity whilst minimising toxicity,” explained Dr Frederick Arce Vargas, Research Associate, UCL Cancer Institute and co-lead author of the study.
Dr Arce Vargas and his colleagues in Professor Sergio Quezada’s lab at UCL Cancer Institute, started studying the CTLA-4 antibody using mouse models. Apart from observing the mechanism that blocks the CTLA-4 signal, they discovered that the antibody was also eliminating regulatory T-cells (Treg cells). This finding was key because Treg cells play a major role in suppressing the body’s immune system, and as a result can promote tumour growth.
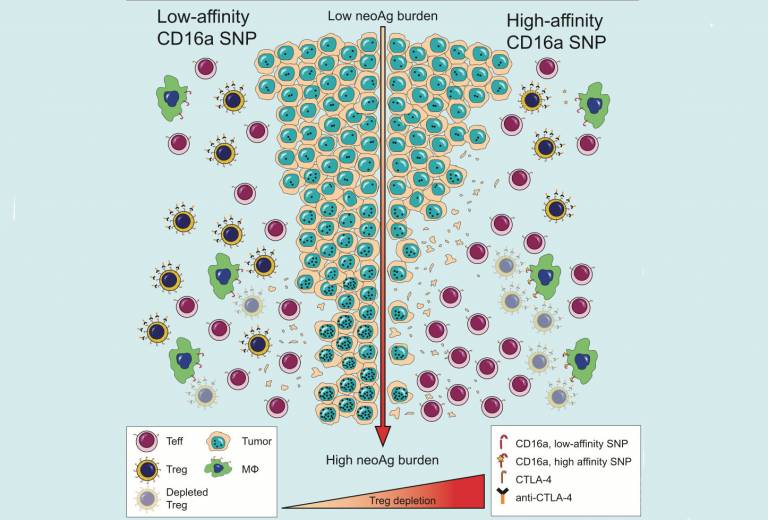
However, the results in lab models cannot always be translated to humans, particularly because the team knew that this elimination of Treg cells was dependent upon Fc-gamma receptors (a protein found on the surface of certain immune cells). Essentially these receptors can detect the Treg cells that have the antibody attached to them and kill them.
To address the question of whether the action of Fc-gamma receptors seen in the lab was relevant in humans, the study team approached it in two ways: “Firstly, we employed a modified mouse model that comprised human Fc-gamma receptors. Using these models we were able to show that the human Fc-gamma receptors were also able to eliminate Treg cells. So this answered a major part of the question. Secondly, we looked back into clinical studies of patients that had been treated with anti-CTLA-4 and the characteristics of Fc-gamma receptors in those patients,” continued Dr Arce Vargas.
The second element of the study was led by Dr Andrew Furness, Royal Marsden NHS Foundation Trust. “It’s important to note that expression of Fc-gamma receptors may vary, depending on the surrounding microenvironment; this is true of tumour relative to blood. In addition, individuals may actually be born with subtle variations that influence the activity of such receptors. A high affinity variant of one such receptor leads to improved engagement with anti-CTLA-4 antibodies and more efficient elimination of Treg cells,” explains Dr Furness.
“When we analysed data from patients that had been treated with anti-CTLA-4 antibodies, patients with the high affinity variant of the Fc-gamma receptor responded much better to therapy than patients with the low affinity variant. However, such activity was limited tumours with a high burden of genetic faults. A hallmark associated with improved infiltration of immune cells within the tumour. The requirement for an inflammed or highly infiltrated tumour microenvironment is key, explaining in part why such antibodies previously demonstrated superior activity in mouse models relative to human subjects. Taking these two parameters together – the high affinity variant Fc-gamma receptor coupled with high levels of genetic faults could feasibly be used as a basis to select which patients are more likely to benefit from anti-CTLA-4 antibodies. For patients lacking these two parameters, tumour response will likely require combination therapy approaches. A final question is the relevance of these observations to drug side effects and this is an area of ongoing research,” says Dr Furness.
Going forward, the study team hope that this research will provide information for future clinical trials to study more groups of patients, and aim to continue their work on understanding the mechanisms underlying the activity of antibodies - work which is fundamental to developing newer and improved immunotherapies that benefit a greater number of patients.
Further information
Research paper: FC Effector Function Contributes to the Activity of Human Anti-CTLA-4 Antibodies. Cancer Cell
 Close
Close

