Chordoma
A rare locally aggressive malignant bone tumour arises in the vertebral bodies, and shows notochordal differentiation. We have one of the largest chordoma tumour banks available for research purposes and lead an NIHR Chordoma observational study across the country. https://chordoma-uk.org/news/show/79/understanding-chordoma-a-national-cohort-study
We were the first to report that this tumour expresses brachyury, a protein only normally seen in the developing embryo (Henderson S. et al., 2005). Through a high through-put drug screen, we identified EGFR inhibitors as the most effective class of drugs able to kill chordoma cancer cells in the laboratory (Scheipl S. et al., 2016). EGFR inhibitors are now being assessed in an international Phase 2 clinical trial (https://www.clinicaltrialsregister.eu/ctr-search/trial/2016-002766-31/GB).
We are employing genome editing techniques, using the CRISPR/Cas9 system, on chordoma cell lines to elucidate the function of a common germline variant that is found in nearly all patients with chordoma and is associated with a six-fold increase in the risk of developing this disease (Pillay N. et al., 2012). This work runs alongside germline genetic analysis of human samples to identify other determinants of disease development to better understand the pathobiology of chordoma.
Chondrosarcoma
Genomic Profiling
This is the second most common primary malignant bone tumour. We were the first to report that cartilaginous tumours commonly harbour an IDH1/2 mutation, and also that this mutation is the cause of Ollier Disease and Maffucci syndrome (Amary F. et al., 2011). In collaboration with the Wellcome Trust Sanger Institute, we have undertaken exome sequencing of 49 primary chondrosarcomas and have and have identified genetic alterations which are known or likely to drive tumour formation (Tarpey S. et al., 2013).
It is challenging to determine how these tumours behave so we are now studying these along with an additional 80 cases which have been submitted to the 100,000 Genomes Projects to determine their impact in clinical behaviour.
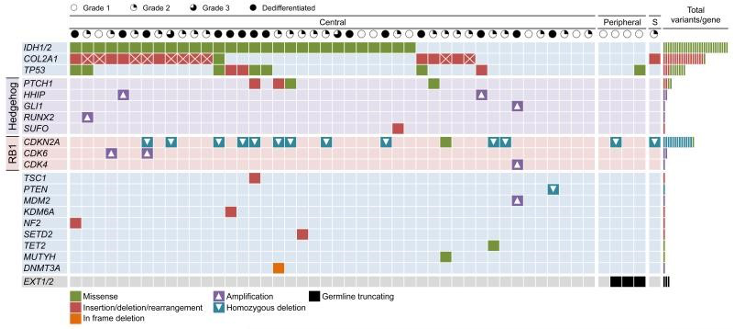
Waterfall plot showing the altered genes and type of mutations identified in the sequenced chondrosarcoma samples (Tarpey P. et al, 2013).
NIHR ctDNA Study
Genomics profiling is complemented by our study which uses circulating tumour DNA (ctDNA) to provide a non-invasive, personalised genomic snapshot of a patients’ tumour. We have already shown that ctDNA is a useful biomarker for tumour diagnosis, prognostication, and residual disease detection in patients with chondrosarcoma (Gutteridge et al., 2017).
We are now determining using a larger cohort of samples if measuring the levels of ctDNA in patients’s blood can predict the grade of a chondrosarcoma more accurately than just looking down a microscope, and whether routine blood tests can be used to monitor disease progression and detect the very early stages of recurrences.
Osteosarcoma
The most common primary malignant bone tumour is a disease that affects all ages but predominantly teenagers and adolescents. It is a heterogeneous disease, both morphologically and genetically. There has been little improvement in clinical outcome for patients with osteosarcoma in the last 40 years.
We are currently undertaking a large multi-omic study of osteosarcoma to determine which patients respond to chemotherapy and to IGF1R inhibitors (Behjati S. et al., 2017).
Furthermore, we are applying ctDNA analysis to monitor disease status, response to neoadjuvant therapies, and to detect residual disease in patients with osteosarcoma.
Nerve Sheath Tumours
Nerve sheath tumours arise from the cells around nerves. Benign nerve sheath tumours (schwannomas and neurofibromas) and malignant (malignant peripheral nerve sheath tumours – MPNST) occur rarely in the general population and commonly in individuals with a disease known as neurofibromatosis type 1. This project is working towards identifying a molecular signature which could distinguish easily between benign and malignant nerve sheath tumours and identifying therapeutic targets for the treatment of malignant disease (Parrinello S et al., 2008; Presneau N. et al., 2012). We are collaborating with Stephan Beck, Professor of Medical Genomics, in the study of methylation of nerve sheath tumours.
 Close
Close



