Gabriella Heller awarded a prestigious Schmidt Science Fellowship to join UCL Biosciences and SMB
16 July 2020

In October 2020, Gabriella Heller, currently the Rosalind Franklin Research Fellow at Newnham College, University of Cambridge, will join the research group of D. Flemming Hansen in the UCL Department of Structural and Molecular Biology, Division of Biosciences as a Schmidt Science Fellow. Gabi plans to use Nuclear Magnetic Resonance (NMR) spectroscopy to characterise the interaction between viral disordered proteins and small molecules.
Gabi completed her MPhil and PhD in the Department of Chemistry at the University of Cambridge under the supervision of Prof. Michele Vendruscolo. There she used computational methods to study the interactions between biomolecules called disordered proteins and drugs. Unlike most proteins with a rigid three-dimensional structure, disordered proteins are extremely dynamic and lack traditional drug binding sites. It is still not clear whether these proteins are viable drug targets and how disordered proteins potentially can bind drugs. Using high-performance computing, Gabi showed how disordered proteins can interact with drug-like small molecules.
As a Schmidt Science Fellow, Gabi will pivot from using computational methods to learning and applying new advanced methods in NMR spectroscopy. Gabi will join the group of Prof. D. Flemming Hansen, a leader in NMR method development. Gabi is particularly interested in characterising the elusive interactions between small molecules and disordered viral proteins experimentally, and she says “The COVID-19 pandemic highlights the urgent need for new antiviral strategies. I am excited to learn more about NMR as it is an extremely powerful experimental tool to characterise disordered proteins and their interactions with small molecules.”
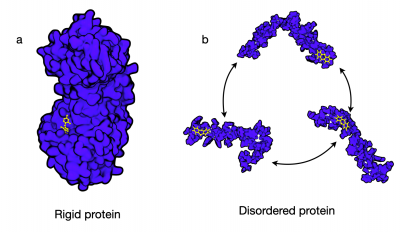
Targeting disordered and flexible proteins represents a novel drug discovery strategy. (a) Conformations of a rigid protein with well-defined grooves and pockets, which can act as binding sites. (b) A disordered protein, which rapidly interconverts between states and generally considered not to have ‘traditional’ drug binding sites.
https://schmidtsciencefellows.org/fellow/gabriella-heller/
@G_T_Heller @SchmidtFellows @DFlemmingHansen @uclbiosciences
 Close
Close

