Centre for Cardiovascular and Metabolic Neuroscience
Metabolic and cardiovascular disease are highly prevalent causes of morbidity and mortality in developed and ageing societies across the globe. The key role of disordered nervous control of metabolism and cardiovascular function is increasingly recognised in the pathogenesis of obesity, diabetes, and circulatory system disease. However, the mechanisms by which autonomic nervous system dysfunction contributes to the development and progression of these common conditions are not well understood.
To facilitate research in this area, the UCL Centre for Cardiovascular and Metabolic Neuroscience (CCMN) was established in 2014. Currently, CCMN brings together several UCL and external research groups that conduct basic and clinical studies directed at understanding the role of neural mechanisms in the development of cardiovascular, cerebrovascular, and metabolic disease.
Current Research
Brain energy metabolism and regulation of cerebral blood flow
The high metabolic rate of the brain, associated with the activities of billions of nerve cells processing information, requires constant and optimal nutrient and oxygen supply, as well as effective elimination of metabolic waste products, ensured by elaborate physiological mechanisms controlling cerebral blood flow. Sustained efficacy of these mechanisms maintains neurologic health and promotes brain longevity. There is growing evidence that reduction in the blood flow to the brain, resulting in impaired delivery of metabolic substrates, damages nerve cells and the connections between them, leading to cognitive decline, and the development of neurodegenerative disease, such as Alzheimer’s disease.

This research is supported by The Wellcome Trust
Autonomic Dysfunction in Diabetes
Diabetes and its co-morbidities such as cardiovascular disease seriously impact on quality of life. Additionally, 10% of the NHS budget is spent on treatment of diabetes alone. Despite the epidemic scale of the problem, clinicians still lack reliable predictors, that help diagnose co-morbidities early, that serve as benchmarks for treatment success, and that determine individual patients' prognosis.
We hypothesize that changes in the autonomic nervous system, leading to imbalances between sympathetic and parasympathetic drives, promote the development of diabetes and related morbidities. The aim of this research is to develop a pre-clinical model of autonomic imbalance that allows us to systemically investigate its potential deleterious consequences.
The role of Glucagon-Like Peptide-1 in the control of energy homeostasis
GLP-1 receptor agonists have recently been shown to be the most efficacious anti-obesity drug to date. However, much of the physiology of GLP-1 remains unclear. GLP-1 is released from the gut into the bloodstream postprandially, but it is also produced by neurons located in the brainstem. Thus GLP-1 acts both as a hormone and as a neurotransmitter. Our work aims at delineating the effects of neuronal GLP-1 from those of gut-released GLP-1, in order to better understand its role in normal feeding behaviour, as well as in obesity and metabolic disease states.
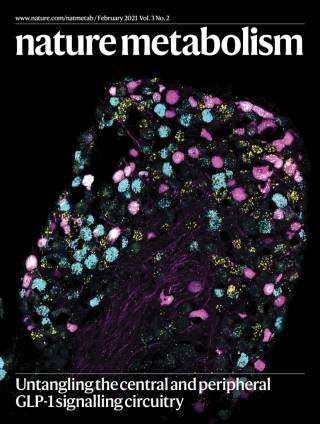
We have recently demonstrated that activation of GLP-1 producing neurons elicits a reduction of food intake beyond that achieved with concomitant administration of a best-in-class GLP-1 receptor agonist highlighting the potential opportunities of combination therapy for the treatment of obesity. This research is primarily supported by the MRC and EFSD.
For further information see:
Autonomic control of the heart during exercise
Our studies aim to determine the physiological principles underlying adaptive and maladaptive alterations in the efficacy of communication between the brain and the heart that develop in response to exercise training and during the progression of cardiovascular disease. Since maintaining physical activity is essential for every aspect of physical, emotional and cognitive health, we also aim to identify how these nervous mechanisms can be switched on to enhance the ability to exercise, increase quality of life and promote cardiovascular health across the lifespan.
This research is supported by The British Heart Foundation.
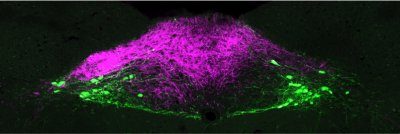
Gut-brain neural circuit dysfunction in obesity
Much of the global population now live in an obesogenic environment, characterised by ready availability of the ultra-processed, high-fat and high-sugar foods which comprise the modern 'Western diet'. This diet is a deleteriously effective trigger of the evolutionarily conserved drive to maximise energy intake during periods of food abundance. While susceptibility to the obesogenic environment is highly variable between individuals, at the population level it drives the chronic overconsumption of food now considered the primary cause of the obesity epidemic. Overconsumption can be caused by impaired function in the neurobiologically-distinct processes of satiety and/or satiation. Satiation processes ultimately elicit meal termination via a poorly understood interaction of neuronal and hormonal signalling pathways between the gastrointestinal tract and the brain - the so-called 'gut-brain axis'.
To prevent the development of obesity in susceptible individuals, and to help people living with obesity to decrease and sustainably maintain their weight, it is vital to understand why dysfunction occurs in the gut-brain pathways controlling meal termination, and how it can be prevented and/or reversed. However, the precise mechanisms underlying the function and dysfunction of these gut-brain satiation circuits are largely unknown. We use transgenic mouse models and systems neuroscience techniques including optogenetics / chemogenetics, electrophysiology, calcium imaging, transcriptomics and lipidomics to interrogate how gut-brain neurocircuits orchestrate meal termination at the cellular, molecular and behavioural levels.
This research is supported by The Wellcome Trust and The Royal Society.
People
| Heads of Centre | |
|---|---|
| Prof Alexander Gourine | |
| Prof Stefan Trapp |
| Research Staff | |
|---|---|
| Dr Qadeer Aziz | |
| Dr Alice Braga | |
| Dr Laura Jones | |
| Dr Daniel Kellett | |
| Mrs Alla Korsak | |
| Dr Anna Roberts | |
| Dr Cecilia Skoug | |
| Dr Shefeeq Theparambil |
| PhD Students | |
|---|---|
| Miss Natalie Figueredo Burgos | |
| Ms Pippa Chapman | |
| Miss Wanqing Jiang | |
| Mr Alexander Mascarenhas |
History
UCL was established in 1826 and soon after under the leadership of William Sharpey became the first centre for experimental studies in Physiology in Britain. Sharpey was truly the father figure of British Physiology, although he was not an experimentalist himself. His Department undertook research in cardiovascular and respiratory physiology and its alumni included most of the major figures in Physiology of the 19th and early 20th centuries who in notable cases went from UCL to establish Physiology in the other major British universities.
These include John Scott Burdon-Sanderson, Michael Foster, Fredrick Bainbridge, Ernest Starling, William Bayliss, Henry Dale and Gleb Anrep amongst others. Perhaps the most eminent physiological experimentalist of this truly distinguished cohort was Starling whose contributions to our understanding of microcirculation, heart function and endocrine system are widely recognized as ground-breaking.
Together with Bayliss he also studied gut motility and provided some of the earliest demonstrations of the role of autonomic innervation in gut function. In the 1920s Starling, together with Anrep, investigated the role of the arterial baroreceptors and came close to establishing the physiological role of the arterial chemoreceptors.
The growth of the Pharmacology Department in the 20th Century and the separation of Biochemistry from Physiology stimulated new areas of research with studies of neurotransmission becoming a major focus in Pharmacology and that of metabolism and nutrition becoming areas of interest in Biochemistry.
These developments were precursors to the major growth in autonomic neuroscience triggered by the arrival of Geoffrey Burnstock's and his appointment as UCL Chair of Anatomy in 1975. His research laid the foundations for our understanding of the peripheral and central nervous control of autonomic function, focusing, in particular, on the key role of purinergic signalling.
At the same time Mike Spyer's group, first at the Royal Free and then at UCL, developed techniques for investigating the role of the CNS mechanisms in controlling circulation and breathing. This research provided major insights into the integrative function of the CNS in autonomic control and revealed intimate links between cardiovascular and respiratory control mechanisms.
On Burnstock's retirement from the Chair of Anatomy in 1997, Burnstock and Spyer established the Autonomic Neuroscience Institute at the Royal Free campus of UCL. This was closed on Burnstock's return to the University of Melbourne in 2017. The new Centre for Cardiovascular and Metabolic Neuroscience led by Alexander Gourine and Stefan Trapp and generously funded by Wellcome, MRC and the BHF aims to build on this proud heritage.
Find out more about the history of Neuroscience research at UCL
 Close
Close


