1. Summary of early stages (stage X - 8)
The following sections contain descriptions of the events of gastrulation and neural induction.
2. Avian gastrulation
(reprinted from Stern, C.D. (2004). Gastrulation in the chick. In: Gastrulation: from cells to embryo. (ed. C.D. Stern). Cold Spring Harbor Press. pp. 219-232. Copyright Claudio D Stern and Cold Spring Harbor Press) - please cite this reference if using any of this information. (if you want to reproduce figures you need to contact both the author and the publishers)
Early stages: blastoderm formation and early polarity
Avian eggs and early embryos differ in several respects from the majority of vertebrates, yet many of the principles governing the early steps of development are very similar. Unlike amphibians and some other embryos, the point of sperm entry is not a crucial determinant of polarity of the early embryo, because avian embryos are highly polyspermic - as many as 5-26 sperm may enter the egg in domestic fowl (chicken) while turkey eggs may be entered by several hundred sperm heads (Waddington et al., 1998; Stepinska and Olszanska, 2003). Cleavage, as in most species containing a large amount of yolk (Arendt and Nübler-Jung, 1999) (Figs. 1-2), is meroblastic - that is, cleavage occurs within a planar disc, and new cell membranes open into the yolk generating small cells in the center and large, yolk-laden, open cells at the periphery (Fig. 2 B, C) (Bellairs et al., 1978). Unfortunately these stages are difficult to study because cleavage occurs while the egg is still within the maternal oviduct and before the shell is deposited (Fig. 1) - in the chicken, laying occurs some 20 hours post-fertilization, when the embryo is a flat disc (blastodisc or blastoderm) containing at least 20,000 cells.
A rule of thumb (von Baer's rule) can help to predict the orientation of the head-tail axis of the embryo from the outside of the egg: with the egg lying along its long axis, and its blunt end to the operator's left, the axis of the embryo will run at right angles to the long egg axis with the head pointing away from the operator. However this rule only applies in about 60-70% of cases.
As in teleosts, which are also meroblastic, maternal determinants are likely to exist also in avian embryos. One such determinant is called δ-ooplasm (or subgerminal ooplasm) - this is contained in the thin, "white" yolk that makes up the latebra and the nucleus of Pander (Fig. 2 A, C). The former is a funnel-like structure extending from just under the blastoderm to the center of the yolk (Callebaut et al., 1998a; Callebaut et al., 1999a; Callebaut et al., 2000a). We do not know the molecular nature or the functions of this ooplasm or whether it plays any role in polarity, although it has been suggested that it determines the position from which the endoblast and Koller's sickle (see below) will form (Callebaut, 1993; Callebaut et al., 1998a; Callebaut et al., 2001).
Avian embryos appear to generate bilateral symmetry under the influence of gravity (Kochav and Eyal-Giladi, 1971; Callebaut, 1978; Eyal-Giladi and Fabian, 1980; Callebaut, 1993; Eyal-Giladi et al., 1994; Callebaut et al., 2001). As the egg descends along the oviduct, it rotates with the blastoderm remaining at an angle of about 45o to the vertical - the lower edge of the blastoderm will become the future head end. However, we are still completely ignorant about the mechanism by which gravity breaks radial symmetry. It was suggested that opposite (upper and lower) poles of the disc are exposed to gravitational forces of different magnitude and that this causes differential amounts of cell shedding (Kochav and Eyal-Giladi, 1971; Eyal-Giladi and Kochav, 1976; Eyal-Giladi and Fabian, 1980; Eyal-Giladi et al., 1994). However, this has never been demonstrated and the alternative hypothesis that rotation exposes the poles of the blastoderm to subgerminal ooplasm to different extents (Callebaut, 1993) seems much more likely.
Importantly, neither gravity nor maternal determinants irreversibly fixes bilateral symmetry until gastrulation starts, because avian embryos are highly regulative - right up to the time of appearance of the primitive streak, blastoderms can be split into several pieces (pie slices) each of which can spontaneously generate a complete embryonic axis (Lutz, 1949; Spratt and Haas, 1960a; Callebaut and Van Nueten, 1995). Therefore, gravity and localized maternal components can, at best, only bias polarity but do not act as definitive determinants.
The blastoderm stage
By the time the egg is laid, the embryo is an almost flat disc in which an inner area pellucida can be distinguished from a more peripheral ring, the area opaca. Closest to the acellular vitelline membrane that envelops the yolk ("dorsal" side), a simple, one-cell-tick epithelium is continuous over both areas (Fig. 3) (Bancroft and Bellairs, 1974; Bellairs et al., 1975). This is the epiblast. At this stage the cells of the epiblast of the two concentric areas are almost indistinguishable morphologically except that at the very edge of the area opaca the cells are flattened and contact the vitelline membrane, against which they will later spread and help expand the blastoderm; the center of the disc is not attached to the membrane. At later stages however cells of the area opaca epiblast become less columnar than those of the area pellucida.
Deep (facing the yolk) to the epiblast the cellular composition is more complex. The area opaca contains several layers of large (up to 150-200μm) yolky cells; those closest to the epiblast are firmly attached to it. This is the germ wall. By contrast, the center of the disc (area pellucida) does not yet contain a continuous cell layer, but is peppered with small islands of about 5-10 cells each. These are also yolky but not as large as the deep part of the area opaca (about 100μm). The islands may arise by a process of polyingression (or shedding) that occurs throughout the area pellucida shortly before laying (Peter, 1938; Kochav et al., 1980; Fabian and Eyal-Giladi, 1981; Eyal-Giladi, 1984), but the fate of the shed cells has never been studied experimentally. The islands will later fuse with each other to generate the primitive endodermal layer, or hypoblast ("entophyll" or "primary hypoblast" in the earlier literature).
Between the area opaca and the area pellucida is a narrow region (known as the marginal zone). The epiblast of this region (to which the term refers) is not distinguishable from other regions of epiblast, except for the expression of Vg1 at its posterior end (posterior marginal zone; see below) (Seleiro et al., 1996; Shah et al., 1997) and a slight gradient of cWnt8C decreasing from posterior to anterior (Skromne and Stern, 2001). The only morphological landmark is that the deep part (germ wall) is not strongly attached to the epiblast, unlike the area opaca. This region is known as the germ wall margin. In carefully dissected blastoderms it forms a lip that protrudes under the area pellucida for a few cell diameters (Stern and Ireland, 1981; Stern, 1990).
The boundary between area pellucida and marginal zone is marked, at the future posterior edge, by a crescent-shaped ridge of small cells, tighly adherent to the epiblast - Koller's sickle (also known as Rauber's sickle) (Koller, 1882; Callebaut and Van Nueten, 1994), which expresses goosecoid (Izpisua-Belmonte et al., 1993). Together, these components define a blastoderm of stage X (Roman numerals from I-XIV are used to classify stages before formation of the primitive streak according to Eyal-Giladi and Kochav, 1976; Arabic numerals from 2 onwards are used for post-streak embryos following Hamburger and Hamilton, 1951).
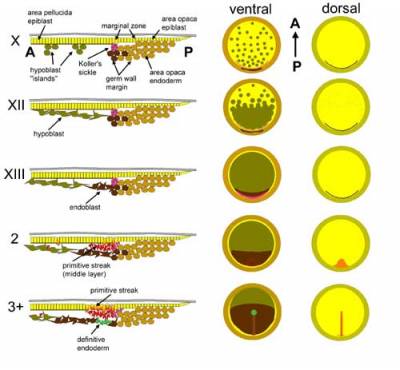
In the following few hours of incubation the islands of hypoblast gradually fuse together, probably by a process of flattening of the cells, which proceeds from posterior to anterior to generate a continuous but relatively loose layer, the hypoblast proper (Vakaet, 1970; Stern, 1990) (see supplementary movie {movie1} and animation {movie2}). This layer covers half of the area pellucida at stage XII and almost all of it at stage XIII (Fig. 3). Shortly after, two changes take place: first, the posterior germ wall margin cells and their progeny start to move centripetally (Stern, 1990) and displace the hypoblast anteriorly; this new layer is the endoblast (or "sickle endoblast" or "secondary hypoblast" in the earlier literature). Hypoblast and endoblast can be distinguished by several markers including goosecoid (in White Leghorns and some other strains), Hex, Hesx1/Rpx, Cerberus/Caronte, Otx2 and Crescent, all of which are expressed in the hypoblast but not in the endoblast (Bachvarova et al., 1998; Foley et al., 2000; Bertocchini and Stern, 2002). The hypoblast is therefore similar to the anterior visceral endoderm (AVE) of the mouse embryo. At the same time, a posterior thickening (the posterior bridge), apparently derived from Koller's sickle appears - this transient structure defines stage XIV, and the primitive streak starts to form immediately thereafter. None of the components of the deep layer (hypoblast, endoblast, germ wall or its margin) contribute to any embryonic tissues - they only generate extraembryonic membranes such as the yolk sac stalk, and later disappear.
Fate maps and cell movements at the blastoderm stage
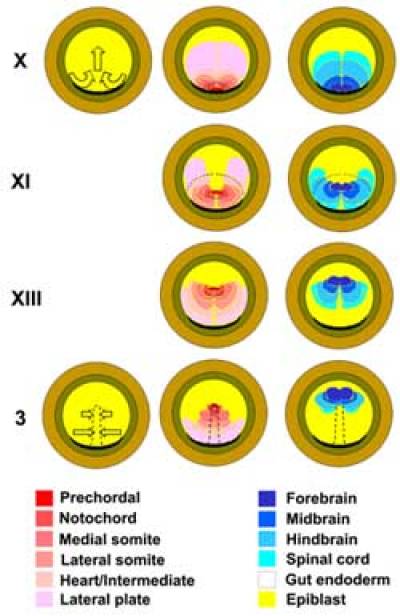
Many authors have constructed fate and specification maps of the epiblast of the chick at the blastoderm stage (Rudnick, 1935, 1938; Hatada and Stern, 1994; Callebaut et al., 1996; Bachvarova et al., 1998). The most detailed ones (Hatada and Stern, 1994) reveal an orderly arrangement of prospective embryonic tissues which gradually changes with time (Fig. 4), due to extensive morphogenetic movements of the epiblast that begin well before primitive streak formation (Gräper, 1929; Vakaet, 1970; Izpisua-Belmonte et al., 1993; Callebaut et al., 1999b; Foley et al., 2000). At stage X, future "dorsal" tissues (prospective organizer and its derivatives: endoderm, prechordal mesoderm, notochord) are found just central to and adjacent to Koller's sickle (Izpisua-Belmonte et al., 1993; Hatada and Stern, 1994; Bachvarova et al., 1998; Streit et al., 2000). These territories quickly move towards the center of the blastoderm, and gradually become replaced in their original position by more lateral regions of epiblast (progressively more "ventral" fates like somite, intermediate mesoderm, etc.) (see supplementary movies: {Weijer.avi} and {normal.avi}). Therefore at stage X the posterior margin of the area pellucida contains a bilateral gradation of dorsal-to-ventral fates, dorsal at the posterior mid-point - subsequent movements "fold" this arrangement into a posterior midline presaging the future primitive streak, so that the most dorsal fates become located most anteriorly along this line (see below). These movements comprise convergence of epiblast towards the posterior mid-point and extension along the midline, but do not seem to occur by the process normally called "convergent extension" in that it is not accompanied by significant cell shape changes. The combination of posterior midpoint convergence and midline extension resembles a Polish dance ("Polonaise") (see supplementary movies: {movie1} and {movie3}), the name given by Gräper (Gräper, 1929) to these epiblast movements after his remarkable stereo-pair time-lapse films of labeled embryos, made as early as 1926.
It is truly remarkable that cells can move horizontally within a relatively tight epithelium, the epiblast. The mechanics of such migration, including the degree to which it is truly "active" (rather than a consequence of mechanical propagation of a remote event like cell loss through ingression), is not yet understood. However, recent observations of living, Bodipy-ceramide chick embryos using two-photon microscopy have started to reveal that individual cells within the epithelium and translocate by "bobbing" up and down, as if each individual cell is a foot in a giant millipede (O. Voiculescu, I.-J. Lau, F. Bertocchini and C.D. Stern, unpublished observations).
We have already described briefly above the movements in the lower layer (Waddington, 1932; Spratt and Haas, 1960b; Vakaet, 1970; Rosenquist, 1972; Stern and Ireland, 1981; Stern, 1990; Bakst et al., 1997; Callebaut et al., 1997a; Bachvarova et al., 1998; Foley et al., 2000; Bertocchini and Stern, 2002). Essentially the hypoblast expands as the islands fuse from posterior to anterior, and the newly-formed hypoblast sheet is then displaced further anteriorly by the incoming endoblast (see supplementary movie {movie1} and animation {movie2}),. The speed at which the hypoblast/endoblast layer spreads is similar to the midline extension in the epiblast (Hatada and Stern, 1994) and recent experiments have shown that there is a causal link: rotation of the deep layer generates a new set of Polonaise movements in the adjacent epiblast (Foley et al., 2000), although the mechanisms by which the two layers communicate are unknown.
These movements also deform Koller's sickle, which appears to be subjected to a large amount of shear. Its anterior (centrally-facing) mid-point will later migrate anteriorly as the primitive streak forms, its lateral extremes converge to the midline, and the posterior aspect (facing the marginal zone) remains posterior and eventually becomes extraembryonic (Izpisua-Belmonte et al., 1993; Bachvarova et al., 1998; Streit et al., 2000).
Cell interactions leading to primitive streak formation
The fact that isolated fragments of blastodiscs can spontaneously initiate axis formation (Lutz, 1949; Spratt and Haas, 1960a) indicates that cell interactions, rather than definitive determinants, must be involved. What are the signals, and where do they come from? Three main sources have been proposed: the hypoblast and/or endoblast, Koller's sickle and the posterior marginal zone (PMZ).
The idea that the hypoblast/endoblast layer regulates the site of primitive streak formation comes from important experiments by Waddington (Waddington, 1932, 1933) in which he demonstrated that rotation of the deep layer (which he called "endoderm") influences the orientation of the primitive streak. When it was rotated by 90o the streak arose from its original site but developed a gradual bend. After 180o rotation of the hypoblast, a few embryos had formed an ectopic streak arising from the opposite (anterior) side. This led Waddington to suggest that the hypoblast layer induces the primitive streak (Waddington, 1933) but he was cautious to avoid ruling out a contribution from cell movements. Subsequent studies were more forceful in proposing induction by the hypoblast (Azar and Eyal-Giladi, 1979, 1981; Mitrani and Eyal-Giladi, 1981; Azar and Eyal-Giladi, 1983; Mitrani et al., 1983) but without providing direct evidence with molecular markers or markers for different cell populations. Later, two studies repeated Waddington's original observations and highlighted the fact that since 90o hypoblast rotations do not induce primitive streak formation from a new site, this is unlikely to be a true inductive event (Khaner, 1995; Foley et al., 2000). Moreover, it was shown (Foley et al., 2000) that the hypoblast layer influences the movements of the overlying epiblast - when rotated, it initates a new set of Polonaise movements at 90o to the original. These compete with the original movements causing the streak to bend, but cells destined for different tissue types do not change their fates.
Recently, a new emphasis has been placed on the second component of the deep layer, the endoblast (Callebaut and Van Nueten, 1995; Callebaut et al., 1998b; Callebaut et al., 1999b; Callebaut et al., 2000b; Bertocchini and Stern, 2002). Specifically (Bertocchini and Stern, 2002), it was shown that complete removal of the hypoblast leads to the formation of multiple streaks at random positions, suggesting that the hypoblast emits an antagonist of axis formation. Analysis of expression patterns and misexpression experiments then suggested that Cerberus, a Nodal antagonist, is responsible. Cerberus is expressed in the hypoblast but not in the endoblast, which is consistent with the fact that the primitive streak starts to form precisely at the time when the hypoblast is displaced away from the posterior edge of the area pellucida by the incoming endoblatst (Bertocchini and Stern, 2002). Finally, it should be mentioned that the hypoblast does have some inducing activity, which can be revealed by assessing the expression of several epiblast genes after grafting a hypoblast ectopically: the homeobox gene Not1/GNOT (Knezevic and Mackem, 2001), and the early "pre-neural" markers ERNI, Sox3 and Otx2 are induced transiently by grafts of the hypoblast to ectopic sites (Foley et al., 1997; Streit et al., 2000) (see Neural induction). The induction of Not1/GNOT may be mediated by retinoids, while induction of ERNI and Sox3 is mediated by FGF. We do not yet know the factors responsible for inducing Otx2.
The second component suggested as playing a role in primitive streak initiation is Koller's sickle (Izpisua-Belmonte et al., 1993; Callebaut and Van Nueten, 1994; Callebaut et al., 1997a; Callebaut et al., 1998b; Callebaut et al., 2003). This structure has been said to give rise to the endoblast (hence the alternative name of "sickle endoblast") and has even been proposed to act as a passage for posterior marginal zone cells from the epiblast to the lower layer (Azar and Eyal-Giladi, 1979; Eyal-Giladi, 1997). However higher resolution fate mapping using different techniques has suggested instead that the sickle contributes cells to the primitive streak itself but not significantly to the endoblast (Izpisua-Belmonte et al., 1993; Bachvarova et al., 1998). Furthermore although grafts of the sickle can indeed generate a second primitive streak upon transplantation, the extensive cellular contribution to the ectopic streak and particularly to definitive (gut) endoderm cannot be dissociated from the inductive effect (Izpisua-Belmonte et al., 1993; Bachvarova et al., 1998).
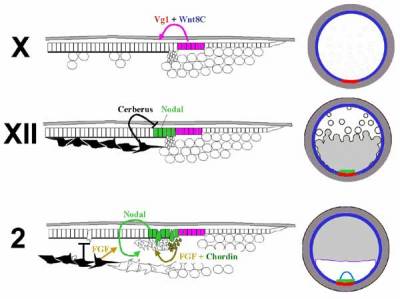
The third and final tissue involved in induction of primitive streak formation is the posterior marginal zone (PMZ) (Spratt and Haas, 1960a; Azar and Eyal-Giladi, 1979; Eyal-Giladi and Khaner, 1989; Khaner and Eyal-Giladi, 1989; Callebaut et al., 1997b; Bachvarova et al., 1998; Bachvarova, 1999; Skromne and Stern, 2001, 2002). Even though its activity as a streak inducer has been challenged (Callebaut et al., 1997b; Callebaut et al., 1998b), there is no question that when grafted into an ectopic position the PMZ is able to induce the formation of a second axis without making a cellular contribution to it, as long as the host is younger than stage XI (Eyal-Giladi and Khaner, 1989; Khaner and Eyal-Giladi, 1989; Bachvarova et al., 1998). These properties of the PMZ have likened it to the amphibian Nieuwkoop center (see Chapters XXX - Keller amphibian; Lemaire Siamois). And like the Nieuwkoop center, whose activity appears to depend upon the overlap of TGFβ and Wnt pathways, the inducing ability of the PMZ can be mimicked by misexpression of cVg1 in regions where Wnt8C is expressed (Seleiro et al., 1996; Shah et al., 1997; Skromne and Stern, 2001, 2002) (Fig. 5). Surprisingly however, unlike PMZ grafts, misexpression of cVg1 in the anterior marginal zone will generate a full axis as late as stage XIII.
In conclusion, all three tissues (hypoblast/endoblast, Koller's sickle and PMZ) proposed to have axis inducing activity do indeed have the ability to influence primitive streak formation. However, their mechanisms of action and relative importance differ. The sickle has inducing ability but this is probably only because it contains some of the cells fated to form Hensen's node (the avian organizer - see below). The earliest influences appear to come from the PMZ, where Vg1 and Wnt activities overlap. Vg1+Wnt induce expression of Nodal in the neighboring area pellucida epiblast, but Nodal can only act (presumably to induce mesendoderm) when the hypoblast has been displaced by the incoming endoblast.
In addition to Vg1+Wnt and Nodal, it is likely that FGFs (emanating from the hypoblast and/or Koller's sickle) (Chapman et al., 2002; Karabagli et al., 2002) also play a role in primitive streak initiation because inhibitors of FGF block this process (Mitrani et al., 1990; Streit et al., 2000) and because misexpression of FGF can generate an ectopic streak (F. Bertocchini, I. Skromne and C.D. Stern, unpublished observations). It is likely that FGF acts in concert with Nodal, as in amphibians (Kimelman and Kirschner, 1987; Cornell and Kimmelman, 1994; LaBonne and Whitman, 1994; Latinkic et al., 1997). Finally, BMP activity also regulates primitive streak formation since ectopic expression of the antagonist Chordin (but not Noggin) is sufficient to induce a streak, even as late as stage 3, and misexpression of BMP4 near the streak causes the streak to disappear (Streit and Stern, 1999). Chordin is normally expressed in Koller's sickle at stages XI-XIV.
Several important questions still remain unanswered. They include: what positions Vg1 expression in the PMZ? What molecular mechanisms underlie regulation when the posterior half of the blastoderm is removed?
Primitive streak formation and elongation
We know surprisingly little about the cellular details of how the primitive streak forms and elongates. Time-lapse films (see movie1, movie3) show that the initial appearance of the streak is extremely rapid - the embryo goes from having no visible axial structures (stage XIV) to developing a triangular, dense streak (stage 2; Fig. 3) in about 30 min, suggesting that primitive streak initiation is accompanied by massive ingression. However, although the basement membrane under the epiblast does partially dissolve during streak formation, this early stage does not involve the loss of epithelial continuity of the epiblast in the region of the forming streak, which happens much later (stage 3+) (Vakaet, 1982; Andries et al., 1983; Sanders and Prasad, 1989; Harrisson et al., 1991). This suggests that the formation of the early, triangular-shape streak is the result of rapid poly-ingression of individual cells through the basal lamina of the epiblast. This process may therefore be analogous to the formation of primary mesenchyme in echinoderms.
Ingression of early, "pioneer" cells from the epiblast to the interior of the embryo can be seen starting as early as stage XII by staining either with the HNK-1 antibody (Canning and Stern, 1988; Stern and Canning, 1990; Canning et al., 2000; Mogi et al., 2000) or for activity of the enzyme Acetylcholinesterase (AChE) (Drews, 1975; Valinsky and Loomis, 1984; Laasberg et al., 1986; Parodi and Falugi, 1989). Indeed, the HNK-1 epitope is carried on a subunit of AChE (Bon et al., 1987), and AChE activity correlates very well with cell ingression and invasiveness in a variety of species (Drews, 1975). It is puzzling that HNK-1/AChE expression differs in different strains of fowl; the salt-and-pepper expression in the epiblast is seen in the Rhode Island Red/Light Sussex cross-breed but not in the more inbred strain, White Leghorn. It also seems clear that HNK-1/AChE expression does not correlate completely with cells destined to form the primitive streak (Cooke, 1993). A few of these cells do ingress but do not contribute to the streak (their fate remains unknown), other HNK-1-postive cells remain in the germ wall margin from where they contribute to the lower layer (Canning and Stern, 1988; Stern and Canning, 1990; Cooke, 1993), and yet others do ingress to the primitive streak but later seem to disappear. Careful fate maps examining the origin of cells that will form the primitive streak have revealed that the definitive primitive streak is largely derived from a relatively small population of epiblast cells local to the site of streak formation and from Koller's sickle (Bachvarova et al., 1998; Wei and Mikawa, 2000).
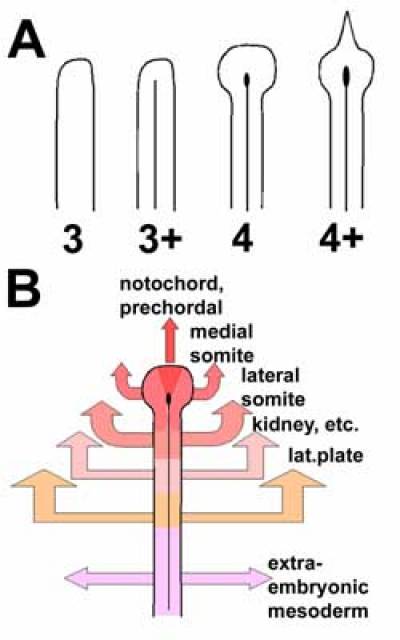
The early triangular streak (stage 2) is made up of a dense accumulation of middle layer cells between epiblast and endoblast (Fig. 3); however it rapidly straightens, to become a mesenchymal rod of parallel sides (stage 3). At this stage there is still no groove in the overlying epiblast and the basement membrane is largely intact. Soon afterwards however two processes take place more or less simultaneously (Vakaet, 1970): the appearance of a longitudinal groove in the epiblast overlying the streak and the start of lateral migration of the mesenchyme of the streak, at right angles to the axis of the streak, to establish the lateral plate. These processes define stage 3+. Since grafts of early (stage 3) streak to a new area can generate an ectopic streak containing an epiblast groove (Vakaet, 1973), and by analogy to the interactions between primary and secondary mesenchyme in echinoderms, it seems likely that the early streak cells induce the formation of a groove (and subsequent invagination) in the overlying epiblast. The signals that mediate this interaction are unknown but FGF and/or Chordin are likely candidates since both can induce a streak at stage 3 (see above).
Stage 4 is marked by the appearance of a distinct bulge at the tip of the streak, encompassing all three layers - this is Hensen's node (Hensen, 1876) (see below). We consider this to be the last phase of the "gastrula stage" in avian embryos - shortly afterwards (stage 4+) a small triangular mass of cells starts to protrude anteriorly from the node (the emerging tip of the head process, which contains precursors for the prechordal mesendoderm - see below). At this time the future neural plate starts to become morphologically and molecularly (Sox2-positive) distinct, and stage 4+ can therefore be considered the beginning of the "neurula stage".
We also know virtually nothing about the mechanics of primitive streak elongation. Time-lapse films reveal that less than 2 hours elapse between the early short streak at stage 2 and the almost fully elongated (1.5mm long) stage 3 streak, making it very unlikely (L. Bodenstein, unpublished computer simulations) that cell division alone is the main force driving this elongation (Wei and Mikawa, 2000). Streak elongation most likely involves a process of cell reorganization and changes in cell shape similar to those seen in amphibian convergent-extension.
Hensen's node
The function of Hensen's node (the avian organizer) will be described elsewhere (see Neural induction). Here we will concentrate on the origin, maintenance and subdivision of the node into different cellular territories.
Various fate mapping techniques have established that Hensen's node arises from two distinct populations of cells (Izpisua-Belmonte et al., 1993; Hatada and Stern, 1994; Bachvarova et al., 1998; Streit et al., 2000; Lawson and Schoenwolf, 2001). One cell population (called "posterior cells" by Streit et al., 2000) resides deep to the epiblast, at the midpoint of Koller's sickle from stage X. It remains in this position until the primitive streak starts to form (stage 2), and then moves anteriorly with the tip of the advancing streak. The second population (called "central cells" by Streit et al., 2000) resides in the epiblast during these early stages - at stage X it is found immediately adjacent to the first population (in the Nodal-expressing territory; see Fig. 5 at stage X). However, the Polonaise movements almost immediately move these cells to the middle of the blastoderm, when these movements stop (stage XIII). As the primitive streak elongates, the posterior cells soon regain contact with the central cells (stages 3-3+). At this time, a morphological node forms (stage 4) and this is accompanied by the acquisition of expression of Sonic hedgehog (Shh) (Levin et al., 1995).
Neither the posterior nor the central cells possess full neural inducing ability by themselves (although posterior cells can induce transient expression of the pre-neural genes Sox3 and ERNI), but acquire this ability when combined (Streit et al., 2000). When transplanted ectopically into the area pellucida of a host embryo, posterior cells can induce neighboring epiblast cells to acquire expression of goosecoid, a marker of the organizer (Izpisua-Belmonte et al., 1993). These findings suggest that during their migration anteriorly from stages 2-3, the posterior cells recruit adjacent epiblast cells to form part of the organizer.
The cellular composition of Hensen's node remains dynamic throughout the early stages of development: even after stage 3+, neighboring epiblast cells migrate to the node, acquire the expression of organizer markers and later migrate out again to emerge in the underlying layers as endoderm, notochord, prechordal mesendoderm or medial somites (Joubin and Stern, 1999). Inducing signals from within the streak (again, Vg1+Wnt, perhaps Nodal) and inhibitory signals from the node itself (ADMP) and from surrounding regions of the blastoderm (BMPs) form a complex network regulating the spatial and temporal expression of node markers including Chordin, Goosecoid, Shh, Not1, HNF3β and others (Joubin and Stern, 1999). These results account for the fact that primitive streak stage embryos from which the node has been extirpated can generate a new node (Grabowski, 1956; Psychoyos and Stern, 1996b; Joubin and Stern, 1999; Yuan and Schoenwolf, 1999).
Despite the dynamic composition of the node at these stages, single cell lineage analysis has suggested that the node also contains a small population of resident cells with stem-cell characteristics (Selleck and Stern, 1991; Selleck and Stern, 1992b). It was proposed that when these cells divide, one daughter remains in the node while the other leaves to contribute to notochord and/or medial somite (Selleck and Stern, 1992b; Stern et al., 1992).
The node is not a uniform structure, either molecularly or by tissue fate. At a molecular level, it displays left-right asymmetry of expression of a number of genes. The earliest of these are Activin receptor IIA (more likely to be a receptor for Nodal) which is expressed on the right and the transcription factor HNF3β which is expressed on the left (Levin et al., 1995; Stern et al., 1995), from stage 3+. By stage 4-4+, while the node starts to develop slight morphological asymmetry, Shh appears on the left, FGF8 on the right and Nodal just to the left of the node (Levin et al., 1995; Dathe et al., 2002). Different regions of the node also give rise preferentially to different structures (Fig. 6), although the boundaries between these fates are not sharp. Specifically, the tip of the node contains mainly prospective notochord, prechordal mesendoderm and floor plate cells, the sides and posterior aspect have mainly prospective medial somite and endodermal precursors (Selleck and Stern, 1991). Transplantation experiments have revealed that the prospective notochord region contains cells that are already committed to this fate while the lateral regions are more plastic (Selleck and Stern, 1992a), but that all regions of the node are indistinguishable in their ability to induce and pattern neural tissue (Storey et al., 1995).
Establishment and subdivision of embryonic endoderm and mesoderm
The study of endoderm formation in avian embryos, as in many other species, has been hindered considerably by the lack of any exclusive, permanent markers for the endoderm lineage. This is even more inconvenient because the endoblast (see above) also lacks specific molecular markers, which makes it very difficult to distinguish these two neighboring tissues except that the endoblast contains typical intracellular inclusions which can be seen under phase contrast in explanted tissues (Stern and Ireland, 1981). It was not until 1953 that Bellairs first recognized that the definitive endoderm is derived from the epiblast via the primitive streak (Bellairs, 1953a, b, 1955, 1957) rather than from the hypoblast layer as was previously thought. The endoderm probably starts to insinuate itself into the lower layer at the early primitive streak stage (stage 2), and this insertion process ends by stage 4 (Vakaet, 1962; Nicolet, 1965; Modak, 1966; Gallera and Nicolet, 1969; Nicolet, 1970; Selleck and Stern, 1991). We are still ignorant about the signals that induce and pattern the endoderm, from where they arise and at what stage, but based on studies in other species it seems likely that Nodal will turn out to play a major role.
By the time the endoderm inserts into the deep layer (stage 3+-4), the original hypoblast cells have become confined to the most anterior part of the blastoderm, a region called the "germinal crescent" because it also contains the primordial germ cells (Ginsburg and Eyal-Giladi, 1986, 1987, 1989; Ginsburg et al., 1989; Tsunekawa et al., 2000). Since the surface of the hypoblast is greater than that of the germinal crescent, the tissue often develops blister-like projections extending ventrally from the surface of the epiblast. The fate of the hypoblast cells after this stage has not been examined thoroughly but it is generally assumed that they contribute to the stalk of the yolk sac. It is equally likely however that a large proportion of hypoblast cells undergo apoptosis, since TUNEL staining at this stage shows heavy labeling in the hypoblast of the germinal crescent (A. Gibson and C.D. Stern, unpublished observations).
The bulk of the middle layer of the stage 3+ primitive streak will give rise to mesoderm: the notochord in the midline with prechordal mesoderm at its tip, the somites, intermediate mesoderm (prospective mesonephric kidney and its duct), heart, lateral plate mesoderm (which includes both embryonic and extraembryonic components). It is important to recognize that the long axis of the primitive streak does not correspond to the future head-tail axis of the embryo but rather to the future dorsoventral axis of the mesoderm: anterior streak (node) gives rise to the most dorsal/axial structures, with more ventral (lateral) structures arising from progressively more posterior streak positions (Schoenwolf et al., 1992; Psychoyos and Stern, 1996a; Sawada and Aoyama, 1999; Freitas et al., 2001; Lopez-Sanchez et al., 2001). This can be understood most easily by looking at the patterns of cell migration from the streak (Figs. 4, 6), and the same relationship is seen in the mouse primitive streak.
It is likely that the major player in imparting specific dorsoventral identity to prospective mesoderm is BMP signaling. The node, which emits BMP antagonists, can transform lateral plate mesoderm into somitic mesoderm (Nicolet, 1968) and this has been shown to be mimicked by Noggin (but not Chordin) (Streit and Stern, 1999). Since Noggin is not expressed in the chick until about stage 4+, it is likely that somite identity is not fixed until after this stage. Indeed, competence for lateral-to-medial transformation and vice-versa remain until at least the early somite stage (Tonegawa et al., 1997; Streit and Stern, 1999; James and Schultheiss, 2003).
Ingression from the epiblast to the deeper layers to form endoderm and the most medial (axial) mesoderm ends around the end of stage 4 (Vakaet, 1962; Nicolet, 1965; Modak, 1966; Gallera and Nicolet, 1969; Nicolet, 1970; Selleck and Stern, 1991; Joubin and Stern, 1999). A recent study has identified a zinc finger transcriptional activator, Churchill, which regulates the cessation of ingression at the primitive streak by activating Sip1, an antagonist of Brachyury (Sheng et al., 2003). This is described in more detail under Neural induction; here we will only point out that expression and activites of Churchill and Sip1 regulate the transition from the end of gastrular ingression to the start of neurulation opposite the anterior levels of the primitive streak.
Time-lapse films show that formation of the head process (the name given to the cranial portion of the notochord, rostral to the future level of the otic vesicle) begins at stage 4+ by forward migration of cells from the node (Spratt, 1947; Bellairs, 1953b). After a short delay (to the end of stage 5) these movements stop and the primitive streak starts to regress (see below), which continues to extend the notochord caudally. Elongation of the notochord appears to include both a process of convergent-extension (as in amphibians and fish) and the gradual deposition of progeny from resident stem cells (see above), but the major ingression movements from the epiblast opposite the anterior primitive streak have ceased by this stage. At more posterior levels, however, ingression to form lateral mesoderm continues for some time.
The emigration of prospective somite and lateral plate mesoderm from the primitive streak is controlled by chemorepulsion by FGF (perhaps FGF8) expressed in the streak (Yang et al., 2002). Yang et al. also proposed that after emerging from the streak, prospective somite tissue is then attracted back to the midline, specifically to FGF4 expressed in the notochord. However, since the entire embryo elongates and narrows at this stage it is difficult to determine whether the migration of somitic mesoderm towards the midline is as active a process as was proposed (Easton et al., 1990). Furthermore, embryos lacking a notochord make a midline row of somites underlying the neural tube, raising the question of what would attract cells to the midline if this model is indeed correct (Stern and Bellairs, 1984).
Ending gastrulation and regression of the primitive streak
The main period of gastrulation is characterized by massive movement of epiblast into the primitive streak to generate mesoderm and endoderm. These movements gradually stop from stages 4-4+ at the most anterior levels of the streak (prospective notochord and medial somite), and progressively more caudally. As mentioned above, the end of ingression through the anterior streak is regulated by Churchill and Sip1 (Sheng et al., 2003). Soon after this (between stages 5-6), the primitive streak starts to regress (see supplementary animation {ChickGastula_animation.avi}),.
Several studies have attempted to establish the main cellular forces driving regression of the primitive streak. The earliest (Spratt, 1947) made the important discovery that shortening of the streak is predominantly a morphological change, rather than a migration of node cells. However, convergent-extension also plays a major role in the process as mentioned earlier (Spratt, 1947; Bellairs, 1963; Lepori, 1966; Stern and Bellairs, 1984; Schoenwolf et al., 1992; Catala et al., 1996; Colas and Schoenwolf, 2001). We still know nothing, however, about the signals that regulate the timing, the speed or the specific changes in cell behavior that control regression.
The tail bud - a continuation of gastrulation?
While regression continues, the deposition of axial and paraxial mesoderm continue as the whole embryo narrows and elongates caudally to generate the tail bud (Sanders et al., 1986; Catala et al., 1996; Knezevic et al., 1998; Charrier et al., 2002). It was therefore proposed that the tail bud is a continuation of the process of gastrulation (Knezevic et al., 1998). While it is true that several processes characteristic of gastrulation do continue in the regressing streak and later in the forming tail bud, other critical processes do not. Specifically, massive ingression of epiblast to form axial tissues (notochord and somites) has ceased (except perhaps at the most caudal end), the formation of new endoderm from the streak has also ended, and regression of the streak is accompanied by cell depletion from this structure. Furthermore the node starts to lose its neural inducing ability just after stage 4 (Dias and Schoenwolf, 1990; Storey et al., 1995) (Neural Induction). Together with the fact that the neural plate starts to elevate at about stage 4+ (Bancroft and Bellairs, 1975), we consider that the end of gastrulation (as a stage) occurs between stages 4 and 4+.
REFERENCES
Andries, L., Vakaet, L. and Vanroelen, C. (1983). The dorsal surface of the animal pole of the just laid quail egg, studied with SEM. Anat Embryol (Berl) 166, 135-147.
Arendt, D. and Nübler-Jung, K. (1999). Rearranging gastrulation in the name of yolk: evolution of gastrulation in yolk-rich amniote eggs. Mech Dev 81, 3-22.
Azar, Y. and Eyal-Giladi, H. (1979). Marginal zone cells--the primitive streak-inducing component of the primary hypoblast in the chick. J Embryol Exp Morphol 52, 79-88.
Azar, Y. and Eyal-Giladi, H. (1981). Interaction of epiblast and hypoblast in the formation of the primitive streak and the embryonic axis in chick, as revealed by hypoblast-rotation experiments. J Embryol Exp Morphol 61, 133-144.
Azar, Y. and Eyal-Giladi, H. (1983). The retention of primary hypoblastic cells underneath the developing primitive streak allows for their prolonged inductive influence. J Embryol Exp Morphol 77, 143-151.
Bachvarova, R. F. (1999). Establishment of anterior-posterior polarity in avian embryos. Curr Opin Genet Dev 9, 411-416.
Bachvarova, R. F., Skromne, I. and Stern, C. D. (1998). Induction of primitive streak and Hensen's node by the posterior marginal zone in the early chick embryo. Development 125, 3521-3534.
Bakst, M. R., Gupta, S. K. and Akuffo, V. (1997). Comparative development of the turkey and chicken embryo from cleavage through hypoblast formation. Poult Sci 76, 83-90.
Bancroft, M. and Bellairs, R. (1974). The onset of differentiation in the epiblast of the chick blastoderm (SEM and TEM). Cell Tissue Res 155, 399-418.
Bancroft, M. and Bellairs, R. (1975). Differentiation of the neural plate and neural tube in the young chick embryo. A study by scanning and transmission electron microscopy. Anat Embryol (Berl) 147, 309-335.
Bellairs, R. (1953a). Studies on the development of the foregut in the chick blastoderm. 1. The presumptive foregut area. J. Embryol. exp. Morph. 1, 115-124.
Bellairs, R. (1953b). Studies on the development of the foregut in the chick blastoderm. 2. The morphogenetic movements. J. Embryol. exp. Morph. 1, 369-385.
Bellairs, R. (1955). Studies on the development of the foregut in the chick embryo. 3. The role of mitosis. J. Embryol. exp. Morph. 3, 242-250.
Bellairs, R. (1957). Studies on the development of the foregut in the chick embryo. 4. Mesodermal induction and mitosis. J. Embryol. exp. Morph. 5, 340-350.
Bellairs, R. (1963). The development of somites in the chick embryo. J. Embryol. exp. Morph. 11, 697-714.
Bellairs, R., Breathnach, A. S. and Gross, M. (1975). Freeze-fracture replication of junctional complexes in unincubated and incubated chick embryos. Cell Tissue Res 162, 235-252.
Bellairs, R., Lorenz, F. W. and Dunlap, T. (1978). Cleavage in the chick embryo. J Embryol Exp Morphol 43, 55-69.
Bertocchini, F. and Stern, C. D. (2002). The hypoblast of the chick embryo positions the primitive streak by antagonizing Nodal signalling. Dev Cell 3, 735-744.
Bon, S., Meflah, K., Musset, F., Grassi, J. and Massoulie, J. (1987). An immunoglobulin M monoclonal antibody, recognizing a subset of acetylcholinesterase molecules from electric organs of Electrophorus and Torpedo, belongs to the HNK-1 anti-carbohydrate family. J Neurochem 49, 1720-1731.
Callebaut, M. (1978). Effects of centrifugation on living oocytes from adult Japanese quails. Anat Embryol (Berl) 153, 105-113.
Callebaut, M., Harrisson, F. and Bortier, H. (2001). Effect of gravity on the interaction between the avian germ and neighbouring ooplasm in inverted egg yolk balls. Eur J Morphol 39, 27-38.
Callebaut, M. and Van Nueten, E. (1994). Rauber's (Koller's) sickle: the early gastrulation organizer of the avian blastoderm. Eur J Morphol 32, 35-48.
Callebaut, M. and Van Nueten, E. (1995). Gastrulation inducing potencies of endophyll and Rauber's sickle in isolated caudocranially oriented prestreak avian blastoderm quadrants (or fragments) in vitro. Eur J Morphol 33, 221-235.
Callebaut, M., Van Nueten, E., Bortier, H. and Harrisson, F. (1999a). Interaction of central subgerminal ooplasm with the elementary tissues (endophyll, Rauber's sickle and upper layer) of unincubated avian blastoderms in culture. Reprod Nutr Dev 39, 589-605.
Callebaut, M., Van Nueten, E., Bortier, H. and Harrisson, F. (2003). Positional information by rauber's sickle and a new look at the mechanisms of primitive streak initiation in avian blastoderms. J Morphol 255, 315-327.
Callebaut, M., van Nueten, E., Bortier, H., Harrisson, F. and van Nassauw, L. (1996). Map of the Anlage fields in the avian unincubated blastoderm. Eur J Morphol 34, 347-361.
Callebaut, M., Van Nueten, E., Bortier, H., Harrisson, F., Van Nassauw, L. and Schrevens, A. (1997a). Spatial relationship between endophyll, primordial germ cells, sickle endoblast and upper layer in cultured avian blastoderms. Reprod Nutr Dev 37, 293-304.
Callebaut, M., Van Nueten, E., Harrisson, F. and Bortier, H. (2000a). Activation of avian embryo formation by unfertilized quail germ discs: comparison with early amphibian development. Reprod Nutr Dev 40, 597-606.
Callebaut, M., van Nueten, E., Harrisson, F., van Nassauw, L. and Bortier, H. (1999b). Endophyll orients and organizes the early head region of the avian embryo. Eur J Morphol 37, 37-52.
Callebaut, M., Van Nueten, E., Harrisson, F., Van Nassauw, L. and Bortier, H. (2000b). Avian junctional endoblast has strong embryo-inducing and -dominating potencies. Eur J Morphol 38, 3-16.
Callebaut, M., Van Nueten, E., Harrisson, F., Van Nassauw, L. and Schrevens, A. (1998a). Induction of (pre) gastrulation and/or (pre) neurulation by subgerminal ooplasm and Rauber's sickle in cultured anti-sickle regions of avian unincubated blastoderms. Eur J Morphol 36, 1-10.
Callebaut, M., van Nueten, E., Harrisson, F., van Nassauw, L., Schrevens, A. and Bortier, H. (1997b). Avian gastrulation and neurulation are not impaired by the removal of the marginal zone at the unincubated blastoderm stage. Eur J Morphol 35, 69-77.
Callebaut, M., Van Nueten, E., Van Nassauw, L., Bortier, H. and Harrisson, F. (1998b). Only the endophyll-Rauber's sickle complex and not cells derived from the caudal marginal zone induce a primitive streak in the upper layer of avian blastoderms. Reprod Nutr Dev 38, 449-463.
Callebaut, M. E. (1993). Early eccentricity in gravitationally oriented quail germs. Eur J Morphol 31, 5-8.
Canning, D. R., Amin, T. and Richard, E. (2000). Regulation of epiblast cell movements by chondroitin sulfate during gastrulation in the chick. Dev Dyn 219, 545-559.
Canning, D. R. and Stern, C. D. (1988). Changes in the expression of the carbohydrate epitope HNK-1 associated with mesoderm induction in the chick embryo. Development 104, 643-655.
Catala, M., Teillet, M. A., De Robertis, E. M. and Le Douarin, M. L. (1996). A spinal cord fate map in the avian embryo: while regressing, Hensen's node lays down the notochord and floor plate thus joining the spinal cord lateral walls. Development 122, 2599-2610.
Chapman, S. C., Schubert, F. R., Schoenwolf, G. C. and Lumsden, A. (2002). Analysis of spatial and temporal gene expression patterns in blastula and gastrula stage chick embryos. Dev Biol 245, 187-199.
Charrier, J. B., Lapointe, F., Le Douarin, N. M. and Teillet, M. A. (2002). Dual origin of the floor plate in the avian embryo. Development 129, 4785-4796.
Colas, J. F. and Schoenwolf, G. C. (2001). Towards a cellular and molecular understanding of neurulation. Dev Dyn 221, 117-145.
Cooke, J. (1993). Expression of the HNK-1 epitope is unaltered among early chick epiblast cells despite behavioral transformation by inducing factors in vitro. Int J Dev Biol 37, 479-486.
Cornell, R. A. and Kimmelman, D. (1994). Activin-mediated mesoderm induction requires FGF. Development 120, 453-462.
Dathe, V., Gamel, A., Manner, J., Brand-Saberi, B. and Christ, B. (2002). Morphological left-right asymmetry of Hensen's node precedes the asymmetric expression of Shh and Fgf8 in the chick embryo. Anat Embryol (Berl) 205, 343-354.
Dias, M. S. and Schoenwolf, G. C. (1990). Formation of ectopic neurepithelium in chick blastoderms: age-related capacities for induction and self-differentiation following transplantation of quail Hensen's nodes. Anat Rec 228, 437-448.
Drews, U. (1975). Cholinesterase in embryonic development. Prog Histochem Cytochem 7, 1-52.
Duval, M. (1889). Atlas d'embryologie. Paris: G. Masson.
Easton, H. S., Bellairs, R. and Lash, J. W. (1990). Is chemotaxis a factor in the migration of precardiac mesoderm in the chick? Anat Embryol (Berl) 181, 461-468.
Eyal-Giladi, H. (1984). The gradual establishment of cell commitments during the early stages of chick development. Cell Differ 14, 245-255.
Eyal-Giladi, H. (1997). Establishment of the axis in chordates: facts and speculations. Development 124, 2285-2296.
Eyal-Giladi, H. and Fabian, B. C. (1980). Axis determination in uterine chick blastodiscs under changing spatial positions during the sensitive period for polarity. Dev Biol 77, 228-232.
Eyal-Giladi, H., Goldberg, M., Refael, H. and Avner, O. (1994). A direct approach to the study of the effect of gravity on axis formation in birds. Adv Space Res 14, 271-279.
Eyal-Giladi, H. and Khaner, O. (1989). The chick's marginal zone and primitive streak formation. II. Quantification of the marginal zone's potencies--temporal and spatial aspects. Dev Biol 134, 215-221.
Eyal-Giladi, H. and Kochav, S. (1976). From cleavage to primitive streak formation: a complementary normal table and a new look at the first stages of the development of the chick. I. General morphology. Dev Biol 49, 321-337.
Fabian, B. and Eyal-Giladi, H. (1981). A SEM study of cell shedding during the formation of the area pellucida in the chick embryo. J Embryol Exp Morphol 64, 11-22.
Foley, A. C., Skromne, I. S. and Stern, C. D. (2000). Reconciling different models of forebrain induction and patterning: a dual role for the hypoblast. Development 127, 3839-3854.
Foley, A. C., Storey, K. G. and Stern, C. D. (1997). The prechordal region lacks neural inducing ability, but can confer anterior character to more posterior neuroepithelium. Development 124, 2983-2996.
Freitas, C., Rodrigues, S., Charrier, J. B., Teillet, M. A. and Palmeirim, I. (2001). Evidence for medial/lateral specification and positional information within the presomitic mesoderm. Development 128, 5139-5147.
Gallera, J. and Nicolet, G. (1969). Le pouvoir inducteur de l'endoblaste presomptif contenu dans la ligne primitive jeune de l'embryon de poulet. J Embryol Exp Morphol 21, 105-118.
Ginsburg, M. and Eyal-Giladi, H. (1986). Temporal and spatial aspects of the gradual migration of primordial germ cells from the epiblast into the germinal crescent in the avian embryo. J Embryol Exp Morphol 95, 53-71.
Ginsburg, M. and Eyal-Giladi, H. (1987). Primordial germ cells of the young chick blastoderm originate from the central zone of the area pellucida irrespective of the embryo-forming process. Development 101, 209-219.
Ginsburg, M. and Eyal-Giladi, H. (1989). Primordial germ cell development in cultures of dispersed central disks of stage X chick blastoderms. Gamete Res 23, 421-427.
Ginsburg, M., Hochman, J. and Eyal-Giladi, H. (1989). Immunohistochemical analysis of the segregation process of the quail germ cell lineage. Int J Dev Biol 33, 389-395.
Grabowski, C. T. (1956). The effects of the excision of Hensen's node on the early development of the chick embryo. J Exp Zool 133, 301-344.
Gräper, L. (1929). Die Primitiventwicklung des Hünchens nach stereokinematographischen Untersuchungen, kontrolliert durch vitale Farbmarkierung und verglichen mit der Entwicklung anderer Wirbeltiere. Arch. EntwMech. Org. 116, 382-429.
Hamburger, V. and Hamilton, H. L. (1951). A series of normal stages in the development of the chick embryo. J Morphol 88, 49-92.
Harrisson, F., Callebaut, M. and Vakaet, L. (1991). Features of polyingression and primitive streak ingression through the basal lamina in the chicken blastoderm. Anat Rec 229, 369-383.
Hatada, Y. and Stern, C. D. (1994). A fate map of the epiblast of the early chick embryo. Development 120, 2879-2889.
Hensen, V. (1876). Beobachtungen über die Befruchtung und Entwicklung des Kaninchens und Meerschweinchens. Z. Anat. EntwGesch. 1, 353-423.
Izpisua-Belmonte, J. C., De Robertis, E. M., Storey, K. G. and Stern, C. D. (1993). The homeobox gene goosecoid and the origin of organizer cells in the early chick blastoderm. Cell 74, 645-659.
James, R. G. and Schultheiss, T. M. (2003). Patterning of the avian intermediate mesoderm by lateral plate and axial tissues. Dev Biol 253, 109-124.
Joubin, K. and Stern, C. D. (1999). Molecular interactions continuously define the organizer during the cell movements of gastrulation. Cell 98, 559-571.
Karabagli, H., Karabagli, P., Ladher, R. K. and Schoenwolf, G. C. (2002). Comparison of the expression patterns of several fibroblast growth factors during chick gastrulation and neurulation. Anat Embryol (Berl) 205, 365-370.
Khaner, O. (1995). The rotated hypoblast of the chicken embryo does not initiate an ectopic axis in the epiblast. Proc Natl Acad Sci U S A 92, 10733-10737.
Khaner, O. and Eyal-Giladi, H. (1989). The chick's marginal zone and primitive streak formation. I. Coordinative effect of induction and inhibition. Dev Biol 134, 206-214.
Kimelman, D. and Kirschner, M. (1987). Synergistic induction of mesoderm by FGF and TGF-beta and the identification of an mRNA coding for FGF in the early Xenopus embryo. Cell 51, 869-877.
Knezevic, V., De Santo, R. and Mackem, S. (1998). Continuing organizer function during chick tail development. Development 125, 1791-1801.
Knezevic, V. and Mackem, S. (2001). Activation of epiblast gene expression by the hypoblast layer in the prestreak chick embryo. Genesis 30, 264-273.
Kochav, S. and Eyal-Giladi, H. (1971). Bilateral symmetry in chick embryo determination by gravity. Science 171, 1027-1029.
Kochav, S., Ginsburg, M. and Eyal-Giladi, H. (1980). From cleavage to primitive streak formation: a complementary normal table and a new look at the first stages of the development of the chick. II. Microscopic anatomy and cell population dynamics. Dev Biol 79, 296-308.
Koller, C. (1882). Untersuchungen über die Blätterbildung im Hühnerkeim. Arch. Mikr. Anat 20, 174-211.
Laasberg, T., Neuman, T. and Langel, U. (1986). Acetylcholine receptors in the gastrulating chick embryo. Experientia 42, 439-440.
LaBonne, C. and Whitman, M. (1994). Mesoderm induction by activin requires FGF-mediated intracellular signals. Development 120, 463-472.
Latinkic, B. V., Umbhauer, M., Neal, K. A., Lerchner, W., Smith, J. C. and Cunliffe, V. (1997). The Xenopus Brachyury promoter is activated by FGF and low concentrations of activin and suppressed by high concentrations of activin and by paired-type homeodomain proteins. Genes Dev 11, 3265-3276.
Lawson, A. and Schoenwolf, G. C. (2001). Cell populations and morphogenetic movements underlying formation of the avian primitive streak and organizer. Genesis 29, 188-195.
Lepori, N. G. (1966). [An analysis of the shortening process of the primitive streak in the blastodisc of the chicken and duck]. Acta Embryol Morphol Exp 9, 61-68.
Levin, M., Johnson, R. L., Stern, C. D., Kuehn, M. and Tabin, C. (1995). A molecular pathway determining left-right asymmetry in chick embryogenesis. Cell 82, 803-814.
Lopez-Sanchez, C., Garcia-Martinez, V. and Schoenwolf, G. C. (2001). Localization of cells of the prospective neural plate, heart and somites within the primitive streak and epiblast of avian embryos at intermediate primitive-streak stages. Cells Tissues Organs 169, 334-346.
Lutz, H. (1949). Sur la production experimentale de la polyembryonie et de la monstruosite double chez les oiseaux. Arch. Anat. Microsc. Morphol. Exp 39, 79-144.
Mitrani, E. and Eyal-Giladi, H. (1981). Hypoblastic cells can form a disk inducing an embryonic axis in chick epiblast. Nature 289, 800-802.
Mitrani, E., Gruenbaum, Y., Shohat, H. and Ziv, T. (1990). Fibroblast growth factor during mesoderm induction in the early chick embryo. Development 109, 387-393.
Mitrani, E., Shimoni, Y. and Eyal-Giladi, H. (1983). Nature of the hypoblastic influence on the chick embryo epiblast. J Embryol Exp Morphol 75, 21-30.
Modak, S. P. (1966). Analyse experimental de l'origine de l'endoblaste embryonnaire chez les oiseaux. Rev. Suisse Zoology 73, 877-908.
Mogi, K., Toyoizumi, R. and Takeuchi, S. (2000). Correlation between the expression of the HNK-1 epitope and cellular invasiveness in prestreak epiblast cells of chick embryos. Int J Dev Biol 44, 811-814.
Nicolet, G. (1965). [Autoradiographic study of the fate of cells invaginating through Hensen's node in the chick embryo at the definitive streak stage]. Acta Embryol Morphol Exp 8, 213-220.
Nicolet, G. (1968). [Role of Hensen's node in the differentiation of somites in birds]. Experientia 24, 263-264.
Nicolet, G. (1970). [An autoradiographic study of the presumptive fate of the primitive streak in chick embryos]. J Embryol Exp Morphol 23, 70-108.
Parodi, M. and Falugi, C. (1989). Effects of acetylcholinesterase specific inhibitors on the development of chick embryos. Boll Soc Ital Biol Sper 65, 839-845.
Peter, K. (1938). Untersuchungen über die Entwicklung des Dotterentoderms. 1. Die Entwicklung des Entoderms beim Hühnchen. Z. mikr. Anat. Forsch. 43, 362-415.
Psychoyos, D. and Stern, C. D. (1996a). Fates and migratory routes of primitive streak cells in the chick embryo. Development 122, 1523-1534.
Psychoyos, D. and Stern, C. D. (1996b). Restoration of the organizer after radical ablation of Hensen's node and the anterior primitive streak in the chick embryo. Development 122, 3263-3273.
Rosenquist, G. C. (1972). Endoderm movements in the chick embryo between the early short streak and head process stages. J Exp Zool 180, 95-103.
Rudnick, D. (1935). Regional restriction of potencies in the chick during embryogenesis. Journal of Experimental Zoology 71, 83-99.
Rudnick, D. (1938). Differentiation in culture of pieces of the early chick blastoderm. The Anatomical Record 70, 351-368.
Sanders, E. J., Khare, M. K., Ooi, V. C. and Bellairs, R. (1986). An experimental and morphological analysis of the tail bud mesenchyme of the chick embryo. Anat Embryol (Berl) 174, 179-185.
Sanders, E. J. and Prasad, S. (1989). Invasion of a basement membrane matrix by chick embryo primitive streak cells in vitro. J Cell Sci 92, 497-504.
Sawada, K. and Aoyama, H. (1999). Fate maps of the primitive streak in chick and quail embryo: ingression timing of progenitor cells of each rostro-caudal axial level of somites. Int J Dev Biol 43, 809-815.
Schoenwolf, G. C., Garcia-Martinez, V. and Dias, M. S. (1992). Mesoderm movement and fate during avian gastrulation and neurulation. Dev Dyn 193, 235-248.
Seleiro, E. A., Connolly, D. J. and Cooke, J. (1996). Early developmental expression and experimental axis determination by the chicken Vg1 gene. Curr Biol 6, 1476-1486.
Selleck, M. A. J. and Stern, C. D. (1991). Fate mapping and cell lineage analysis of Hensen's node in the chick embryo. Development 112, 615-626.
Selleck, M. A. J. and Stern, C. D. (1992a). Commitment of mesoderm cells in Hensen's node of the chick embryo to notochord and somites. Development 114, 403-415.
Selleck, M. A. J. and Stern, C. D. (1992b). Evidence for stem cells in the mesoderm of Hensen's node and their role in embryonic pattern formation. In Formation and differentiation of early embryonic mesoderm., (ed. R. Bellairs E. J. Sanders and J. W. Lash), pp. 23-31. New York: Plenum Press.
Shah, S. B., Skromne, I., Hume, C. R., Kessler, D. S., Lee, K. J., Stern, C. D. and Dodd, J. (1997). Misexpression of chick Vg1 in the marginal zone induces primitive streak formation. Development 124, 5127-5138.
Sheng, G., Dos Reis, M. and Stern, C. D. (2003). Churchill, a zinc finger transcriptional activator, regulates the transition from gastrulation to neurulation. Cell 115, 603-613.
Skromne, I. and Stern, C. D. (2001). Interactions between Wnt and Vg1 signalling pathways initiate primitive streak formation in the chick embryo. Development 128, 2915-2927.
Skromne, I. and Stern, C. D. (2002). A hierarchy of gene expression accompanying induction of the primitive streak by Vg1 in the chick embryo. Mech Dev 114, 115-118.
Spratt, N. T. (1947). Regression and shortening of the primitve streak in the explanted chick blastoderm. J Exp Zool 104, 69-100.
Spratt, N. T. and Haas, H. (1960a). Integrative mechanisms in development of the early chick blastoderm. I. Regulative potentiality of separated parts. J Exp Zool 145, 97-137.
Spratt, N. T. and Haas, H. (1960b). Morphogenetic movements in the lower surface of the unincubated and early chick blastoderm. Journal of Experimental Zoology 144, 139-157.
Stepinska, U. and Olszanska, B. (2003). DNase I and II present in avian oocytes: a possible involvement in sperm degradation at polyspermic fertilisation. Zygote 11, 35-42.
Stern, C. D. (1990). The marginal zone and its contribution to the hypoblast and primitive streak of the chick embryo. Development 109, 667-682.
Stern, C. D. and Bellairs, R. (1984). The roles of node regression and elongation of the area pellucida in the formation of somites in avian embryos. J Embryol Exp Morphol 81, 75-92.
Stern, C. D. and Canning, D. R. (1990). Origin of cells giving rise to mesoderm and endoderm in chick embryo. Nature 343, 273-275.
Stern, C. D., Hatada, Y., Selleck, M. A. and Storey, K. G. (1992). Relationships between mesoderm induction and the embryonic axes in chick and frog embryos. Development Supplement 1992, 151-156.
Stern, C. D. and Ireland, G. W. (1981). An integrated experimental study of endoderm formation in avian embryos. Anat Embryol 163, 245-263.
Stern, C. D., Yu, R. T., Kakizuka, A., Kintner, C. R., Mathews, L. S., Vale, W. W., Evans, R. M. and Umesono, K. (1995). Activin and its receptors during gastrulation and the later phases of mesoderm development in the chick embryo. Dev Biol 172, 192-205.
Storey, K. G., Selleck, M. A. and Stern, C. D. (1995). Neural induction and regionalisation by different subpopulations of cells in Hensen's node. Development 121, 417-428.
Streit, A., Berliner, A., Papanayotou, C., Sirulnik, A. and Stern, C. D. (2000). Initiation of neural induction by FGF signalling before gastrulation. Nature 406, 74-78.
Streit, A. and Stern, C. D. (1999). Mesoderm patterning and somite formation during node regression: differential effects of chordin and noggin. Mech Dev 85, 85-96.
Tonegawa, A., Funayama, N., Ueno, N. and Takahashi, Y. (1997). Mesodermal subdivision along the mediolateral axis in chicken controlled by different concentrations of BMP-4. Development 124, 1975-1984.
Tsunekawa, N., Naito, M., Sakai, Y., Nishida, T. and Noce, T. (2000). Isolation of chicken vasa homolog gene and tracing the origin of primordial germ cells. Development 127, 2741-2750.
Vakaet, L. (1962). Some data concerning the formation of the definitive endoblast in the chick embryo. Journal of Embryology and Experimental Morphology 10, 38-57.
Vakaet, L. (1970). Cinephotomicrographic investigations of gastrulation in the chick blastoderm. Arch Biol (Liege) 81, 387-426.
Vakaet, L. (1973). [Inductions by the nodus posterior of the primitive streak of birds]. C R Seances Soc Biol Fil 167, 1053-1055.
Vakaet, L. (1982). [Experimental study of the ingression during gastrulation in the chick blastoderm]. Verh K Acad Geneeskd Belg 44, 419-437.
Valinsky, J. E. and Loomis, C. (1984). The cholinergic system of the primitive streak chick embryo. Cell Differ 14, 287-294.
Waddington, C. H. (1932). Experiments on the development of chick and duck embryos cultivated in vitro. Phil. Trans. Roy. Soc. Lond. B 221, 179-230.
Waddington, C. H. (1933). Induction by the endoderm in birds. W. Roux Arch. EntwMech. Org 128, 502-521.
Waddington, D., Gribbin, C., Sterling, R. J., Sang, H. M. and Perry, M. M. (1998). Chronology of events in the first cell cycle of the polyspermic egg of the domestic fowl (Gallus domesticus). Int J Dev Biol 42, 625-628.
Wei, Y. and Mikawa, T. (2000). Formation of the avian primitive streak from spatially restricted blastoderm: evidence for polarized cell division in the elongating streak. Development 127, 87-96.
Yang, X., Dormann, D., Munsterberg, A. E. and Weijer, C. J. (2002). Cell movement patterns during gastrulation in the chick are controlled by positive and negative chemotaxis mediated by FGF4 and FGF8. Dev Cell 3, 425-437.
Yuan, S. and Schoenwolf, G. C. (1999). Reconstitution of the organizer is both sufficient and required to re- establish a fully patterned body plan in avian embryos. Development 126, 2461-2473.
FIGURE LEGENDS
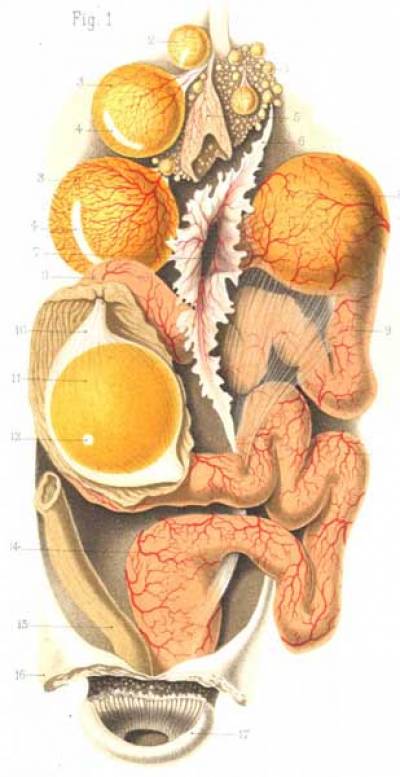
Fig. 1. Formation of the hens' egg and its descent along the maternal oviduct. From (Duval, 1889).
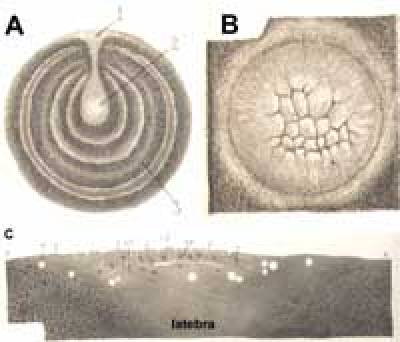
Fig. 2. Cleavage in the chick embryo. A. A section through the yolk reveals concentric rings (3) of dense (darker) and white (lighter) yolk. Under the blastoderm (1), a sub-blastodermic space filled with white yolk forms a funnel (the latebra) that extends deep into the center of the yolk mass, where it forms a small cavity, the Nucleus of Pander (2). B. Cleavage in the chick embryo is meroblastic: the cleavage planes open into the surrounding yolk mass. C. A section through the blastoderm and the surrounding yolk reveals the subgerminal cavity and latebra. All three figures dapted from (Duval, 1889).
Fig. 3. Stages of gastrulation in the chick embryo. Stages are indicated on the left: Roman numerals denote pre-primitive streak stages (X-XIV) (Eyal-Giladi and Kochav, 1976) and Arabic numbers the stages from the appearance of the primitive streak (stage 2 onwards) (Hamburger and Hamilton, 1951). The left column shows diagrammatic mid-sagittal sections through part of the blastoderm, posterior to the right. The middle column depicts whole embryos viewed from the ventral (endodermal) side, and the rightmost column embryos viewed from the dorsal (epiblast) aspect. Grey: vitelline membrane; Yellow: epiblast; Dark green: hypoblast; Light brown: area opaca endoderm (germ wall and its margin); Dark brown: endoblast; Red: primitive streak mesendoderm; Purple: Koller's sickle; Bright green: definitive (gut) endoderm.
Fig. 4. Summary fate maps of the epiblast and cell movement patterns at different stages. The two diagrams on the left hand column show the major movements in the epiblast: Polonaise movements before primitive streak formation, and convergence of the epiblast to the streak (which is strongest posteriorly) during gastrulation. The middle column of diagrams summarize the locations of territories of cells that give rise to different mesodermal tissues and the gut endoderm. The rightmost column summarizes the locations of subdivisions of the neural plate. The dashed line at stage XI indicates the most anterior extent of spread of the hypoblast layer at this stage, and the dashed outline at stage 3 is the profile of the primitive streak.
Fig. 5. Molecular interactions implicated in the initiation of primitive streak formation, shown at three successive stages (indicated on the left), in sections (left column) and in whole mounts (right). At stage X, Vg1 (red; expressed in the posterior marginal zone) cooperates with Wnt8C (blue; expressed throughout the marginal zone) to induce Nodal (bright green) in the neighboring epiblast of the area pellucida. However, Nodal cannot act further because it is inhibited by Cerberus (black) produced by the underlying hypoblast (stage XII). Shortly before primitive streak formation (stages XIV-2), the displacement of the hypoblast by the non-Cerberus-expressing endoblast allows Nodal signaling to act. Nodal, in cooperation with FGF (light brown; emanating from the hypoblast and from Koller's sickle) and Chordin (dark green; produced by Koller's sickle) then induce ingression of cells from the epiblast to form the primitive streak. (Based on data from several sources, mainly (Skromne and Stern, 2001; Bertocchini and Stern, 2002).
Fig. 6. Fate maps of the primitive streak and Hensen's node. A. Morphology of the anterior tip of the primitive streak at different stages: stage 3 (no groove, parallel sides), 3+ (groove, parallel sides), 4 (distinct node), 4+ (incipient head process, elongated pit). B. Fates and movement patterns of mesoderm emerging from different portions of the streak at stage 4. Note that the anterior-posterior axis of the streak corresponds not to the head-tail axis of the embryo but rather to the mediolateral (axial-lateral, or dorsoventral) axis of the mesodermal organs. Based on data from several sources, mainly (Selleck and Stern, 1991; Psychoyos and Stern, 1996a).
3. Neural induction
(reprinted from Stern, C.D. (2004). Neural induction. In: Gastrulation: from cells to embryo. (ed. C.D. Stern). Cold Spring Harbor Press. pp. 419-432. Copyright Claudio D Stern and Cold Spring Harbor Press) - please cite this reference if using this information. (if you want to reproduce figures you need to contact both the author and the publishers)
What is neural induction?
Embryonic induction has been defined by Gurdon as "... an interaction between one (inducing) tissue and another (responding) tissue, as a result of which the responding tissue undergoes a change in its direction of differentiation" (Gurdon, 1987). Neural induction is therefore the process by which cells acquire a neural fate in response to appropriate signals during development or after embryonic manipulations that bring two dissimilar cell types together. During normal vertebrate development, neural induction is generally believed to occur around the time of gastrulation, directed at least in part by signals emanating from a special region of the embryo: "the organizer". The organizer resides in the embryonic shield of teleosts, the dorsal lip of the blastopore in amphibians and the tip of the primitive streak (Hensen's node) in amniotes. This Chapter takes an historical approach to trace the development of our understanding of this process at the cellular and molecular levels.
Early history

The concept of induction originates in von Baer's work (von Baer, 1828) and was further developed at the turn of the 20th Century notably by Curt Herbst (Oppenheimer, 1991). But the concept of neural induction really
evolved from the pioneering experiments of Warren Lewis (Lewis, 1907) and the better known work of Hans Spemann and Hilde Mangold (Spemann and Mangold, 1924; Hamburger, 1988; De Robertis and Aréchaga, 2001) (Figs. 1, 2). As part of an effort to resolve an on-going controversy about whether embryos are "regulative" or "mosaic", Lewis found that transplantation of the dorsal lip of the blastopore of Rana to an ectopic position caused a second axis to form. However, he interpreted this as self-differentiation of the graft and it was not until Spemann's use of interspecies grafts between three differently pigmented species of newts (Triturus taeniatus, T. cristatus and T. alpestris) (Spemann, 1921; Spemann and Mangold, 1924), allowing the cells of the donor and host to be distinguished, that it could be clearly concluded that this was an example of an inductive interaction. Spemann and Mangold termed the dorsal lip of the blastopore "the organizer", because it could direct the formation of a coherently organized, ectopic axis from cells whose fate was other than to form axial structures.
It took only a few years for these findings to be extended to other vertebrates, including amniotes: first to avian species (chick and duck) (Hunt, 1929; Waddington, 1930, 1932; Waddington, 1933b) and shortly afterwards to mammalian embryos (rabbit), by interspecies grafts in all combinations (Waddington, 1932, 1934; Waddington, 1936, 1937). In all these cases the primitive streak, and specifically Hensen's node at its anterior end, were found to contain the "organizer activity".
The ability of the organizer to induce a nervous system is coupled with its ability to pattern the induced structures, the property that led to its name. Anteroposterior patterning is discussed elsewhere in this book (Fraser and Stern, 2004); suffice it to say here that several models have been proposed to account for this activity of the organizer, the main ones being the "head/trunk/tail" model most clearly formulated by Otto Mangold (Mangold, 1933), which proposes the existence of separate inducing activities for the head, trunk and tail portions of the axis, and the "activation/transformation" model of Nieukwoop (Nieuwkoop et al., 1952; Nieuwkoop and Nigtevecht, 1954), which proposes that the nervous system that is initially induced is of "anterior" (forebrain) character and that later signals "transform" parts of it to more caudal fates. Currently there is evidence both for and against both opposing models and the issue has not yet been fully resolved (see Stern, 2001). The rest of this essay will concentrate on neural induction proper - the cellular and molecular mechanisms leading to the specification of neural fate regardless of its rostrocaudal character.
Seven fruitless decades
Following the identification of the organizer and of neural induction, the hunt began for the "organizing principles". Spemann himself favored a vitalistic explanation, while several laboratories (most notably those of Holtfreter and O. Mangold and later Tiedemann and Grunz in Germany, Toivonen and Saxén in Finland, Dorothy and Joseph Needham and Waddington in England, Nakamura and Yamada in Japan and Brachet in Belgium) embarked on trying to identify a chemical inducer (Holtfreter, 1933; Waddington, 1933a; Holtfreter, 1934; Needham et al., 1934; Spemann, 1938; Toivonen, 1938; Chuang, 1939, 1940; Toivonen, 1940; Waddington, 1940; Holtfreter, 1945; Saxén and Toivonen, 1962; Toivonen et al., 1975; Rollhauser-ter Horst, 1977b, a; Saxen, 1980; Chen and Solursh, 1992; reviewed in Nakamura and Toivonen, 1978). Early indications for a steroid, then for various protein or RNA extracts, led to transient flurries of excitement, which quickly waned as a result of the discovery that numerous "heterologous", or non-specific inducers (including killed organizers, high or low pH, alcohol, histological dyes, …) were just as effective as an organizer graft in inducing a second axis in amphibians. Essentially no progress was made until well into the 1990s.
A turning point: BMP antagonism and the "default model"
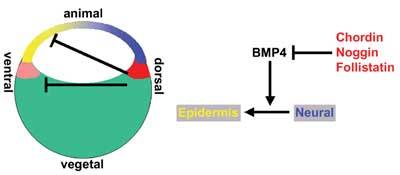
Several seemingly unrelated observations gradually led to a new concept, commonly known as "the default model" for neural induction (Hemmati-Brivanlou and Melton, 1997) (Fig. 3). First, several groups had observed that in amphibians, dissociation of gastrula-stage animal caps into single cells for a short time before reaggregating them again leads to the formation of neural tissue (Born et al., 1989; Godsave and Slack, 1989; Grunz and Tacke, 1989; Sato and Sargent, 1989; Saint-Jeannet et al., 1990). A few years later, it was found that misexpression of a dominant-negative "activin"-receptor (it was later discovered that this construct inhibits several TGFβ-related factors) into Xenopus embryos not only blocks mesoderm formation but also generates ectopic neural tissue (Hemmati-Brivanlou and Melton, 1992, 1994). At about the same time, it was discovered that BMP4 is a ventralizing factor in Xenopus (Dale et al., 1992; Jones et al., 1992). Several of these authors speculated that neural induction might be induced by removal of some inhibitory substance (Hemmati-Brivanlou and Melton, 1994), but direct evidence was still lacking.
Soon, three genes encoding proteins with neuralizing activity were isolated and found to be expressed in the organizer: Noggin (Smith and Harland, 1992; Lamb et al., 1993; Smith et al., 1993), Follistatin (Hemmati-Brivanlou et al., 1994) and Chordin (Sasai et al., 1994; Sasai et al., 1995). But it took several other findings before the connections were established firmly: the turning points were the finding that BMP4 is an effective inhibitor of neural fate while promoting epidermal differentiation (even in dissociated cells) (Hawley et al., 1995; Wilson and Hemmati-Brivanlou, 1995) and the observations that all three neuralizing/dorsalizing proteins, Noggin, Chordin and Follistatin, are binding partners and antagonists of BMP signaling (De Robertis and Sasai, 1996; Piccolo et al., 1996; Zimmerman et al., 1996; Fainsod et al., 1997). The Drosophila homolog of Chordin (Short gastrulation, or Sog) also binds and inactivates the BMP4 homolog Decapentaplegic (Dpp) and vertebrate Chordin can even rescue sog mutants (Francois and Bier, 1995; Holley et al., 1995; Schmidt et al., 1995; Biehs et al., 1996; De Robertis and Sasai, 1996; Ferguson, 1996).
Together, these findings led to the "default model" (Hemmati-Brivanlou and Melton, 1997), which proposes that cells within the ectoderm layer of the frog gastrula have an autonomous tendency to differentiate into neural tissue. This tendency is inhibited by bone morphogenetic proteins - in particular, BMP4, which acts as an epidermal inducer (Fig. 3).
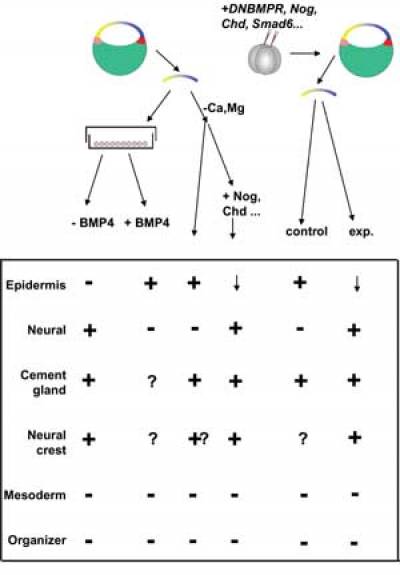
Consistent with this model (Fig. 4), neuralization does not occur after dissociation of animal caps obtained from embryos previously injected with RNA encoding effectors of BMP4 (Msx1, Smad1 or Smad5; Suzuki et al., 1997a; Suzuki et al., 1997b; Wilson et al., 1997), consistent with the view that the neural pathway is inhibited by an endogenous BMP-like activity. Moreover, the expression pattern of BMP4 in Xenopus conforms to its proposed anti-neural function: in the early gastrula, BMP4 transcripts are widely expressed in the entire ectoderm and then clear from the future neural plate at the time when the organizer appears (Fainsod et al., 1994). Transcription of BMP RNA is maintained by the activity of BMP protein (Biehs et al., 1996), which accou
nts for the disappearance of BMP4 and -7 expression from the vicinity of the organizer (which secretes BMP inhibitors) at the gastrula stage (Fainsod et al., 1994; Hawley et al., 1995).
The model is further supported by the effects of treatments that inhibit the BMP signaling pathway. Animal caps cut from embryos injected with either RNA encoding dominant-negative receptors that bind BMPs (Hemmati-Brivanlou and Melton, 1994; Xu et al., 1995), or non-cleavable forms of BMP4 or -7 (Hawley et al., 1995), or antisense BMP4 RNA (Sasai et al., 1995) adopt a neural fate instead of epidermis (Fig. 4). Finally, Chordin and Noggin protein can neuralize isolated animal caps (provided that these have been exposed briefly to low Ca++/Mg++-medium - effectively a partial dissociation, although the rationale given for this is that it helps the protein penetrate between the cells).
In addition to its role in neural induction, the organizer can also pattern the mesoderm at the gastrula stage ("dorsalization"). This activity can also be attributed to BMP inhibition. BMPs can modify dorsal mesoderm to give ventral cell types (Dale et al., 1992; Fainsod et al., 1994; Jones et al., 1996), while their inhibitors can generate notochord and muscle from ventral mesoderm (Smith et al., 1993; Sasai et al., 1994; Tonegawa et al., 1997; Tonegawa and Takahashi, 1998; Streit and Stern, 1999b). BMP inhibitors can also regulate the dorsoventral polarity of the whole embryo before gastrulation. For example, UV irradiated embryos lack dorsoventral polarity and fail to gastrulate, but can be rescued fully by injection of RNA encoding any of the BMP inhibitors: the blastopore (dorsal) will form close to the site of injection (Smith and Harland, 1992; Sasai et al., 1994).
In addition to Chordin, Noggin and Follistatin, other secreted molecules that antagonize BMP signaling have been found and several of these are expressed in, or close to, the organizer. These include Cerberus (Bouwmeester et al., 1996; Belo et al., 1997), Gremlin, Dan and Drm (Hsu et al., 1998; Pearce et al., 1999; Dionne et al., 2001; Eimon and Harland, 2001; Khokha et al., 2003), Ogon/Sizzled (Wagner and Mullins, 2002; Yabe et al., 2003b) and Twisted gastrulation (Chang et al., 2001).
Finally, a recent study by the De Robertis lab demonstrated that organizer activity, or at least dorsalization, requires functional Chordin (Oelgeschlager et al., 2003). Together, these findings provide compelling evidence that BMPs and their modulation by endogenous inhibitors are involved in the activities of the organizer, including the establishment of neural and non-neural domains in Xenopus. This model is very attractive both because of its simplicity and also because it provides the first truly coherent model to explain neural induction since the discovery of the organizer by Spemann and Mangold.
More complexity?
What followed was perhaps a little reminiscent of the events in the 1940s that eventually led to a temporary loss of interest in identifying neural inducers (see above) - a flurry of papers reporting neural inducing activity of a variety of other molecules (Otte et al., 1988; Otte et al., 1990; Otte et al., 1991; Bolce et al., 1992; Kengaku and Okamoto, 1995; Lamb and Harland, 1995; Sokol et al., 1995; Witta et al., 1995; Hansen et al., 1997; Rodriguez-Gallardo et al., 1997; Xu et al., 1997; Alvarez et al., 1998; Barnett et al., 1998; Mariani and Harland, 1998; Storey et al., 1998; Baker et al., 1999; Hongo et al., 1999; Kato et al., 1999; Leclerc et al., 1999; Matsuo-Takasaki et al., 1999; Beanan et al., 2000; Fekany-Lee et al., 2000; Hardcastle et al., 2000; Ishimura et al., 2000; Strong et al., 2000; Kim and Nishida, 2001; Pera et al., 2001; Sullivan et al., 2001; Wessely et al., 2001; Borchers et al., 2002; Peng et al., 2002; Tsuda et al., 2002; Osada et al., 2003; Pera et al., 2003; Yabe et al., 2003a). Some of these clearly act by inhibition of the BMP pathway at some level, while others do not obviously act that way. Many of those that do not, however, seem to act by regulating the pattern of the whole embryo at earlier stages of development. Misexpression experiments conducted by injection of RNA at early cleavage stages gives results that are difficult to interpret in terms of whether the encoded protein acts directly (as a true neural inducer) or through prior induction of a cell fate that can emit an inducer.
To some extent this explains some contradictory findings. For example, the Wnt pathway has been reported to act as a neural inducer by some (Sokol et al., 1995; Baker et al., 1999; Wessely et al., 2001) but to inhibit neural induction by others (Wilson et al., 2001). The difference could be accounted for if at early stages Wnt dorsalizes the embryo (inducing tissues with organizer properties), while at later stages Wnt activity somehow antagonizes other signals from the organizer (Bainter et al., 2001; Wilson and Edlund, 2001). It becomes important to use reagents that work in a cell-autonomous manner and to express them in a stage- and position-controlled way (as appropriate to the specific inductive event being studied).
A more complex literature surrounds the role of FGF signaling in neural induction. A first study implicated this pathway indirectly when Suramin (which inhibits FGF among other related proteins) was found to block neural induction in Xenopus (Grunz, 1992). Later, several labs found that FGF can induce neural tissue under certain circumstances (Lamb and Harland, 1995; Rodriguez-Gallardo et al., 1997; Alvarez et al., 1998; Barnett et al., 1998; Storey et al., 1998; Hongo et al., 1999; Hardcastle et al., 2000; Ishimura et al., 2000; Wilson et al., 2000; Kim and Nishida, 2001; Hudson et al., 2003), while other studies suggested that FGF is not a sufficient signal for neural induction (Amaya et al., 1991; Cox and Hemmati-Brivanlou, 1995; Kroll and Amaya, 1996; Holowacz and Sokol, 1999; Ribisi et al., 2000; Pownall et al., 2003). One explanation for this discrepancy is the observation that different FGF receptors are required to mediate different activities of FGF: FGFR1 is required for the mesoderm-inducing function of FGF, while FGFR4 appears to mediate its role in neuralization (Hardcastle et al., 2000; Umbhauer et al., 2000). The involvement of FGF signals in neural induction will be discussed further below.
FGF, Wnt and BMPs in neural induction
Despite the obvious attraction of the default model, several observations in different organisms do not fit its proposals so neatly. In Xenopus, inhibition of FGF signaling by a dominant-negative version of the FGF-receptor-1 (XFD) blocks the neuralizing activity of both Noggin and Chordin (Launay et al., 1996; Sasai et al., 1996). Furthermore, mere cutting of the animal cap activates MAP kinase by phosphorylation, at least transiently (LaBonne and Whitman, 1997), which could explain the finding made by several labs that "control" animal caps express markers for the cement gland (Fig. 4). These observations suggest that FGF signaling is required for neural induction in addition to BMP inhibition.
In chick (Figs. 5-6), the patterns of expression of components of the BMP pathway do not agree with the model: Chordin continues to be expressed in organizer at stages when this has lost its neural inducing activity, and Noggin and Follistatin are not expressed in the organizer at the appropriate stages at all, while BMP4 and BMP7 are expressed only weakly (if at all) in the ectoderm before neural induction begins, and their expression increases at the border of the neural plate starting from the end of gastrulation (stage 4) (Streit et al., 1998). Moreover, misexpression of Chordin or Noggin in competent epiblast does not neuralize the epiblast (Streit et al., 1998; Streit and Stern, 1999b; Linker et al., 2004) (Fig. 6) and dissociation of the epiblast leads to differentiation of muscle rather than neurons (George-Weinstein et al., 1996). In zebrafish, neither Noggin nor Follistatin are expressed in the organizer (Bauer et al., 1998) and Chordin (chordino) mutants, although ventralized, still have a neural plate (Hammerschmidt et al., 1996a; Hammerschmidt et al., 1996b; Kishimoto et al., 1997; Schulte-Merker et al., 1997; Bauer et al., 1998). In mouse, BMP4 mutants are uninformative (the embryos die too early with mesoderm and other generalized defects (Winnier et al., 1995), but BMP2 and BMP7 mutants lack an early neural phenotype (Dudley et al., 1995; Zhang and Bradley, 1996) and Chordin-, Noggin- and even Chordin-Noggin double mutants have a respectable neural plate (Brunet et al., 1998; McMahon et al., 1998; Bachiller et al., 2000). In the urochordate Ciona, FGF signaling through the MEK pathway but not BMP inhibition appears to be responsible for neural induction (Darras and Nishida, 2001; Hudson and Lemaire, 2001; Kim and Nishida, 2001; Bertrand et al., 2003; Hudson et al., 2003).
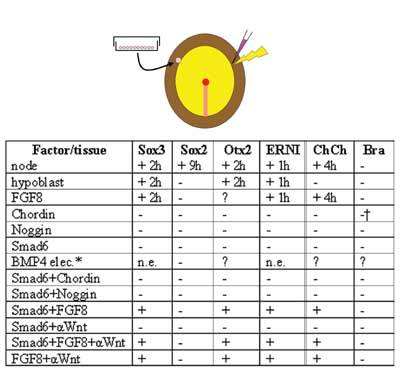
FGF signaling now appears to be a prerequisite for neural induction but this step occurs (or at least begins) very ear
ly, before gastrulation (Streit et al., 2000; Wilson et al., 2000). However, FGF does not appear to be a sufficient or direct neural inducer in vertebrates (Streit et al., 2000). Wilson and colleagues (Wilson et al., 2001) suggested that FGF only shows neural inducing activity when Wnt signaling is also blocked, and proposed that there are two divergent pathways both involving FGF: for "medial epiblast cells" (prospective neural plate), FGF signaling alone is sufficient to repress the BMP pathway and thus cause neuralization. For "lateral epiblast cells" (prospective epidermis), both FGF signaling and Wnt inhibition are required to block the BMP pathway and neuralize (Wilson and Edlund, 2001; Wilson et al., 2001). It has been proposed (Bainter et al., 2001; Wilson and Edlund, 2001) that the critical event involves regulation of BMP4 transcription. Most of these experiments have been conducted using explants, and in our own experiments in intact embryos we are unable to neuralize competent epiblast by any combination of FGF, Wnt antagonists and/or BMP antagonists at any stage of development (Streit et al., 2000; Linker et al., 2004). Furthermore, we find that misexpression of the intracellular BMP antagonist Smad6 in chick embryos is not sufficient to cause neuralization of competent epiblast even when combined with secreted BMP antagonists (Chordin and Noggin), FGF, secreted Wnt antagonists (NFz8, Dkk1 and crescent) and a multifunctional antagonist (Cerberus) (Linker et al., 2004). We therefore believe that not all the required signals have been identified, and that although down-regulation of BMP is almost certainly involved in the specification of the neural plate, this is not a sufficient signal, even in combination with FGF and Wnt inhibition.
Looking upstream from a critical promoter
To date, therefore, we have not yet arrived at a full understanding of the molecular signals that trigger the acquisition of neural fate by the ectoderm. One reason for this may be the diversity of approaches used in different "model" systems, and that many of the approaches used have not taken full account of the issue of developmental timing, which is particularly important when studying molecules with multiple, and sometimes opposing functions at different times. One might however gain further insight by changing the viewpoint to the promoter of a target gene. This has recently been attempted for the first time by analysis of the Sox2 promoter, which is a good marker for committed, developing neural plate in the chick (unlike the mouse where the early functions of Sox3 and Sox2 appear to have been exchanged). Kondoh and colleagues have identified many enhancers both upstream and downstream of the cSox2 gene, which are conserved in mouse and human (Uchikawa et al., 2003). Two of these enhancers, N1 and N2, appear to be responsible for the onset of expression of Sox2 in the early neural plate - they contain binding sites for several identified transcription factors, including a Sox-related protein (perhaps Sox3, which in the chick is expressed earlier in a similar domain to Sox2), TCF/LEF (Wnt pathway), homeodomain-containing proteins and an E-box sequence shown to be a target of Sip1/δEF1 (Verschueren et al., 1999). Sip1 was recently identified as a target of the zinc finger protein Churchill, and morpholino-mediated down-regulation of Churchill function leads to loss of the neural plate, while misexpression of Churchill can confer or maintain the competence of epiblast to neural inducing signals from the node (Sheng et al., 2003) (see Avian gastrulation).
A view from the streak/blastopore
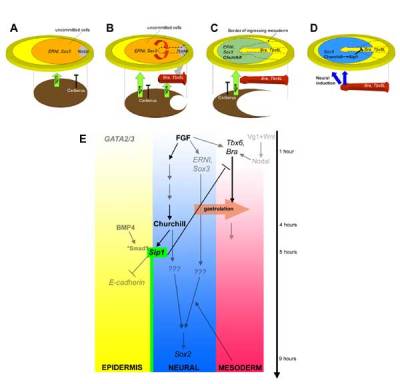
Churchill was first isolated from a molecular screen designed to identify genes that a
re regulated by 5 hours of signaling from a graft of the organizer, Hensen's node, in the chick embryo (Sheng et al., 2003). This was done because previous studies had revealed that 5 hours' exposure to a node are required for epiblast cells to become sensitive to Chordin misexpression (by maintaining Sox3 expression, which is otherwise only transiently induced by a node graft) (Streit et al., 1998). Churchill is expressed in the prospective neural plate from the late gastrula stage and thereafter persists in the forming neural plate in a pattern similar to that of Sox2. Both a node graft and misexpression of FGF induce Churchill expression in about 4 hours, as expected from the screen (Sheng et al., 2003) (Fig. 7). Churchill misexpression close to the streak of the chick embryo or the blastopore of frog embryos causes down-regulation of the mesodermal marker Brachyury; however, Churchill is a transcriptional activator, which suggested that one of its targets may be a repressor of Brachyury. A selection strategy and gel mobility shift assays identified the sequence CGGGRR as a binding target of Churchill, and analysis of the putative regulatory regions of Sip1 identified numerous occurrences of this sequence. Indeed Sip1 is expressed identically to Churchill and morpholino-knockdown of the latter causes loss of Sip1 expression (Sheng et al., 2003). Sip1 is a good candidate to mediate the down-regulation of Brachyury by Churchill, since it this is one of its known functions (Verschueren et al., 1999; Lerchner et al., 2000; Papin et al., 2002).
Misexpression of Churchill near the primitive streak causes not only loss of Brachyury but also a failure of cells to continue to ingress through the streak to form mesendoderm. Since Churchill begins to be expressed at about the time this ingression stops through the anterior primitive streak (see Avian gastrulation), this raised the possibility that one of its functions may be to end the process of gastrulation to keep some epiblast cells on the surface. This hypothesis is supported by the finding that morpholino down-regulation of Churchill at the late gastrula/early neurula stage causes cells to continue to ingress through the streak, and ectopic contribution to the mesoderm rather than neural plate. This effect can be rescued by co-electroporation of either Churchill or of its target Sip1 (Sheng et al., 2003). Thus, Churchill regulates the end of ingression through the streak as well as the competence of the cells that express it to respond to neural inducing signals from the node, raising the possibility that this is a critical protein in the neural induction process, and particularly in regulating the transition from gastrulation to neurulation. This finding drew attention to the rather overlooked fact that at the gastrula stage, the prospective mesoderm territory lies adjacent to the prospective neural plate in all vertebrate classes, and to the possibility that during normal development the decisions leading to the acquisition of neural fate involves a switch between these two identities in addition to the choice between epidermis and neural plate, as suggested by the Spemann/Mangold transplantation experiment and its equivalent in other species. One possibility, therefore, is that two separate decisions lead to the establishment of the neural plate: one (a decision between mesendoderm and neural fates) at the medial edge of the neural plate, involving Churchill and Sip1 medially, which prevent further gastrular ingression, and the other (neural versus epidermis) at its lateral/anterior edges, which could involve inhibition of BMP signaling.
A view from the border
The same screen that led to the identification of Churchill also identified another early response to an organizer graft: ERNI (Early Response to Neural Induction) (Streit et al., 2000). Like Churchill, this is expressed in the prospective neural plate but its expression begins much earlier, before gastrulation. At the end of gastrulation ERNI is down-regulated starting from the center of the neural domain until it is expressed only at the neural-epidermis border, and then it disappears from this domain after stage 7. Also like Churchill, ERNI is induced by a graft of the node and by FGF, but this induction is much more rapid (just 1 hour). Analysis of the expression of different FGFs and comparison with these early "pre-neural" markers suggested that ERNI and Sox3 are first induced before gastrulation, by FGF emanating from either the hypoblast or from prospective organizer cells at the posterior end of the blastodisc or both (Streit et al., 2000). Indeed, transplantation of either of these tissues can induce ERNI and Sox3 just like a node or FGF, and blocking the FGF pathway abolishes their induction by any of these tissues. These and other findings (Streit and Stern, 1999a) led to the view that an early response to neural induction (and FGF signaling) is the specification of a region with "border-like" character, which is responsive to BMP and its antagonists (Streit et al., 1998; Streit and Stern, 1999a, b). Subsequent events confine these properties exclusively to the future neural/epidermal border, as the neural plate proper becomes insensitive to BMP during gastrulation. By stage 3+-4, the only region sensitive to BMP signaling is the border itself: up-regulation of BMP here moves the border towards the midline (but only by a modest amount), narrowing the neural plate, while down-regulation of BMP at the border widens the neural plate (but again only slightly). Sip1 was first identified by its interaction with phosphorylated Smad1, a target of BMP (Verschueren et al., 1999), raising the possiiblity that Churchill, through Sip1, contributes to sensitizing cells to BMP antagonists after 5 hours' exposure to FGF.
The situation in amphibians may not be different from that in birds. Based on at least some fate maps from early (32-64-cell) stages in both Xenopus (Jacobson and Hirose, 1981; Moody, 1987; Moody and Kline, 1990) and in other amphibians (Moury and Jacobson, 1989, 1990; Saint-Jeannet and Dawid, 1994; Delarue et al., 1997), the most animal blastomeres (A2 and A3) will contribute progeny to the neural crest (i.e. the border of the neural plate). Since most injections designed to test the ability of BMP antagonists and other factors to induce a neural plate are placed in the animal pole, it is likely that they target, at least in part, what may be the most sensitive region: the neural/epidermal border. It is also possible that the observations that cutting an animal cap activates MAPK as well as inducing expression of cement gland markers (the cement gland is part of the anteriormost border of the neural plate; see above) are causally connected. In agreement with this view, injection of the dowstream inhibitory component of the BMP pathway, Smad6 in Xenopus at the 32-cell stage causes axis duplications and/or expansion of the neural plate when placed into the A1-A3 animal blastomeres, but no ectopic neural plate when placed into the most ventral, A4 animal blastomere (Delaune et al., 2004; Linker et al., 2004). Together these observations raise the possibility that inhibition of the BMP pathway may only be effective in generating ectopic neural plate (expansion of the endogenous neural plate) within or close to the border between neural and epidermal territories, but not within a region wholly destined to give rise to epidermis. The results also point to the border of the neural plate as a special region, distinct from both neural plate and epidermis.
The timing of neural induction
Organizer grafts are technically easiest after the start of gastrulation, when the blastopore, primitive streak or shield can be identified morphologically. Experiments in which the stage of the host and donor embryos were varied in different vertebrate classes established that neural induction is likely to end by the end of the gastrula stage. For example, in the chick, a Hensen's node taken from an embryo up to stage 4 can induce a complete nervous system but older donors gradually lose their inducing ability, while hosts rapidly lose their competence between stages 4 and 4+ (Damas, 1947; Gallera and Ivanov, 1964; Gallera, 1971; Dias and Schoenwolf, 1990; Storey et al., 1992; Streit et al., 1997). Experiments such as these have suggested that neural induction by the organizer is likely to end by the end of gastrulation, but do not give insight into when the process starts. As mentioned above, at least some of the early signals may be present and active before the start of gastrulation (Streit et al., 2000; Wilson et al., 2000). At this time, a number of "pre-neural" genes are expressed in the epiblast (including ERNI, Sox3 and Otx2), in a fairly broad domain which includes but is not restricted to the future neural plate. Some cells expressing all three markers are destined to contribute to mesendoderm as well as neural/epidermis border and some epidermis. Further signals and other refining mechanisms are required downstream of this initial "pre-neural" specification to commit cells to a neural fate.
In the chick, grafts of the hypoblast (which expresses both FGF8 and the Wnt antagonists Dkk-1, crescent and Cerberus) can induce all three "pre-neural" genes, but only transiently (Foley et al., 2000; Streit et al., 2000). It has been suggested (Stern, 2001) that signals from the node or from its derivatives like the head process/notochord and/or the prechordal mesendoderm may be required to stabilize this early expression and to drive cells to Sox2 expression and commitment to a neural plate fate. The hypoblast is equivalent (in terms of fate as well as expression of various markers) to the Anterior Visceral Endoderm (AVE) of the mouse (see Avian gastrulation), which has been shown to be required for normal development of the mouse forebrain (Thomas and Beddington, 1996; Beddington and Robertson, 1998, 1999). However, it is important to point out that neither the chick hypoblast nor the mouse AVE are equivalent to Spemann's organizer in that neither can induce the formation of a neural plate/axis when grafted to an ectopic site. In the mouse, it was shown that forebrain induction requires a combination of the AVE, the "early gastrula organizer" (EGO - which may contain some of the precursors of the later node) as well as the appropriate responding part of the epiblast (prospective forebrain) (Tam and Steiner, 1999).
The findings that grafts of a mouse node to a lateral site induce a nervous system lacking the most rostral structures (Beddington, 1994) and that homozygous HNF3β mutants, which lack a node (Klingensmith et al., 1999), still have a fairly acceptable neural tube, have been used by some to argue that the node is not essential for neural induction. Indeed, it is clear that the most rostral portions of the neural plate (prospective forebrain) are never adjacent to the mature node in either mouse or chick embryos. However, the former finding can be explained because mouse embryos are very small and it is virtually impossible to find a site to graft the node allowing a complete axis to form without fusing with the host, and the latter can be explained by the possibility that although no morphological node or its derivatives form, some of its properties are also expressed by other tissues. Furthermore, by the time a "node" can be defined morphologically in the mouse, the embryos are starting to produce a head process; chick nodes at the equivalent stage (4+/5) have already lost their ability to induce the most rostral parts of the CNS (Dias and Schoenwolf, 1990; Storey et al., 1992). Taking this evidence together, the most parsimonious view is therefore that during normal development, signals from the AVE and/or other areas initiate some of the earliest events of neural induction, but are not sufficient. As development proceeds to gastrulation, "maintenance" signals as well as regional identity are imparted by the node and/or its derivatives (prechordal and chordal tissues) (Stern, 2001). However, the node itself (provided that it is taken from an embryo at the full primitive streak stage but before any prechordal/head process cells emerge) contains sufficient signals to induce a complete axis when grafted far enough from the host neural plate. In the chick this can be done in the inner third of the area opaca, which only has extraembryonic fate (Gallera, 1971; Dias and Schoenwolf, 1990; Storey et al., 1992; Streit et al., 1998); in the mouse (and perhaps also in Xenopus), it is impossible to find a site far enough from the host neural plate to avoid recruitment of host neural plate cells.
Conclusions
Although very substantial progress has been made in understanding neural induction since the pioneering experiments of the 1920s, there are still substantial gaps in our knowledge of both the cellular and molecular aspects of this important process. It is now becoming more likely than rather than a single inducing factor, a whole cascade of molecular events and converging pathways are required to specify the neural plate, and that different mechanisms may contribute to define this territory in different embryonic locations at different times. Our view is that a full understanding will only come when the embryological/cellular processes can be fully correlated with their molecular basis - much the same as what Wolpert may have had in mind when criticizing excessive emphasis on signaling events ("inductions") at the expense of understanding the resulting patterns: "… induction and its related concepts, which have so dominated embryological thinking, have completely obscured the problems of pattern formation by emphasizing the information coming from some other tissue rather than the response in the tissue which gives rise to the pattern … {a} failure of inductive theory to consider the problem of spatial organization" (Wolpert, 1970; quoted from Horder, 2001).
References
Alvarez, I. S., Araujo, M. and Nieto, M. A. (1998). Neural induction in whole chick embryo cultures by FGF. Dev Biol 199, 42-54.
Amaya, E., Musci, T. J. and Kirschner, M. W. (1991). Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell 66, 257-270.
Bachiller, D., Klingensmith, J., Kemp, C., Belo, J. A., Anderson, R. M., May, S. R., McMahon, J. A., McMahon, A. P., Harland, R. M., Rossant, J. and De Robertis, E. M. (2000). The organizer factors Chordin and Noggin are required for mouse forebrain development. Nature 403, 658-661.
Bainter, J. J., Boos, A. and Kroll, K. L. (2001). Neural induction takes a transcriptional twist. Dev Dyn 222, 315-327.
Baker, J. C., Beddington, R. S. and Harland, R. M. (1999). Wnt signaling in Xenopus embryos inhibits bmp4 expression and activates neural development. Genes Dev 13, 3149-3159.
Barnett, M. W., Old, R. W. and Jones, E. A. (1998). Neural induction and patterning by fibroblast growth factor, notochord and somite tissue in Xenopus. Dev Growth Differ 40, 47-57.
Bauer, H., Meier, A., Hild, M., Stachel, S., Economides, A., Hazelett, D., Harland, R. M. and Hammerschmidt, M. (1998). Follistatin and noggin are excluded from the zebrafish organizer. Dev Biol 204, 488-507.
Beanan, M. J., Feledy, J. A. and Sargent, T. D. (2000). Regulation of early expression of Dlx3, a Xenopus anti-neural factor, by beta-catenin signaling. Mech Dev 91, 227-235.
Beddington, R. S. (1994). Induction of a second neural axis by the mouse node. Development 120, 613-620.
Beddington, R. S. and Robertson, E. J. (1998). Anterior patterning in mouse. Trends Genet 14, 277-284.
Beddington, R. S. and Robertson, E. J. (1999). Axis development and early asymmetry in mammals. Cell 96, 195-209.
Belo, J. A., Bouwmeester, T., Leyns, L., Kertesz, N., Gallo, M., Follettie, M. and De Robertis, E. M. (1997). Cerberus-like is a secreted factor with neuralizing activity expressed in the anterior primitive endoderm of the mouse gastrula. Mech Dev 68, 45-57.
Bertrand, V., Hudson, C., Caillol, D., Popovici, C. and Lemaire, P. (2003). Neural tissue in ascidian embryos is induced by FGF9/16/20, acting via a combination of maternal GATA and Ets transcription factors. Cell 115, 615-627.
Biehs, B., Francois, V. and Bier, E. (1996). The Drosophila short gastrulation gene prevents Dpp from autoactivating and suppressing neurogenesis in the neuroectoderm. Genes Dev 10, 2922-2934.
Bolce, M. E., Hemmati-Brivanlou, A., Kushner, P. D. and Harland, R. M. (1992). Ventral ectoderm of Xenopus forms neural tissue, including hindbrain, in response to activin. Development 115, 681-688.
Borchers, A. G., Hufton, A. L., Eldridge, A. G., Jackson, P. K., Harland, R. M. and Baker, J. C. (2002). The E3 ubiquitin ligase GREUL1 anteriorizes ectoderm during Xenopus development. Dev Biol 251, 395-408.
Born, J., Janeczek, J., Schwarz, W. and Tiedemann, H. (1989). Activation of masked neural determinants in amphibian eggs and embryos and their release from the inducing tissue. Cell Differ Dev 27, 1-7.
Bouwmeester, T., Kim, S.-H., Sasai , Y., Lu, B. and De Robertis, E. M. (1996). Cerberus is a head-inducing secreted factor expressed in the anterior endoderm of Spemann's Organizer. Nature 382, 595-601.
Brunet, L. J., McMahon, J. A., McMahon, A. P. and Harland, R. M. (1998). Noggin, cartilage morphogenesis, and joint formation in the mammalian skeleton. Science 280, 1455-1457.
Chang, C., Holtzman, D. A., Chau, S., Chickering, T., Woolf, E. A., Holmgren, L. M., Bodorova, J., Gearing, D. P., Holmes, W. E. and Brivanlou, A. H. (2001). Twisted gastrulation can function as a BMP antagonist. Nature 410, 483-487.
Chen, Y. and Solursh, M. (1992). Comparison of Hensen's node and retinoic acid in secondary axis induction in the early chick embryo. Dev Dyn 195, 142-151.
Chuang, H.-H. (1939). Induktionsleistungen vond frischen und gekochten Otganteilen (Niere, Leber) nach ihrer Verpflanzung in Explantate und versdhiedene Wirtsregionen von Tritonkeimen. Arch. EntwMech. Org. 139, 556-638.
Chuang, H.-H. (1940). Weitere Versuch über die Veränderung der Induktionsleistungen von gekochten Organteilen. Arch. EntwMech. Org. 140, 25-38.
Cox, W. G. and Hemmati-Brivanlou, A. (1995). Caudalization of neural fate by tissue recombination and bFGF. Development 121, 4349-4358.
Dale, L., Howes, G., Price, B. M. J. and Smith, J. C. (1992). Bone morphogenetic protein 4: a ventralizing factor in early Xenopus development. Development 115, 573-585.
Damas, H. (1947). Effet de la suspension précoce du flux inducteur sur la détermination du neurectoblaste médullaire. Arch. Biol. (Liège) 58, 15-57.
Darras, S. and Nishida, H. (2001). The BMP/CHORDIN antagonism controls sensory pigment cell specification and differentiation in the ascidian embryo. Dev Biol 236, 271-288.
De Robertis, E. M. and Aréchaga, J. (2001). The Spemann-Mangold organizer. Int J Dev Biol 45, 1-378.
De Robertis, E. M. and Sasai, Y. (1996). A common plan for dorso-ventral patterning in Bilateria. Nature 380, 37-40.
Delarue, M., Saez, F. J., Johnson, K. E. and Boucaut, J. C. (1997). Fates of the blastomeres of the 32-cell stage Pleurodeles waltl embryo. Dev Dyn 210, 236-248.
Delaune, E., Lemaire, P. and Kodjabachian, L. (2004). ??? (to follow). (submitted).
Dias, M. S. and Schoenwolf, G. C. (1990). Formation of ectopic neurepithelium in chick blastoderms: age-related capacities for induction and self-differentiation following transplantation of quail Hensen's nodes. Anat Rec 228, 437-448.
Dionne, M. S., Skarnes, W. C. and Harland, R. M. (2001). Mutation and analysis of Dan, the founding member of the Dan family of transforming growth factor beta antagonists. Mol Cell Biol 21, 636-643.
Dudley, A. T., Lyons, K. M. and Robertson, E. J. (1995). A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev 9, 2795-2807.
Eimon, P. M. and Harland, R. M. (2001). Xenopus Dan, a member of the Dan gene family of BMP antagonists, is expressed in derivatives of the cranial and trunk neural crest. Mech Dev 107, 187-189.
Fainsod, A., Deissler, K., Yelin, R., Marom, K., Epstein, M., Pillemer, G., Steinbeisser, H. and Blum, M. (1997). The dorsalizing and neural inducing gene follistatin is an antagonist of BMP-4. Mech Dev 63, 39-50.
Fainsod, A., Steinbeisser, H. and De Robertis, E. M. (1994). On the function of BMP-4 in patterning the marginal zone of the Xenopus embryo. Embo J 13, 5015-5025.
Fekany-Lee, K., Gonzalez, E., Miller-Bertoglio, V. and Solnica-Krezel, L. (2000). The homeobox gene bozozok promotes anterior neuroectoderm formation in zebrafish through negative regulation of BMP2/4 and Wnt pathways. Development 127, 2333-2345.
Ferguson, E. L. (1996). Conservation of dorsal-ventral patterning in arthropods and chordates. Curr Opin Genet Dev 6, 424-431.
Foley, A. C., Skromne, I. S. and Stern, C. D. (2000). Reconciling different models of forebrain induction and patterning: a dual role for the hypoblast. Development 127, 3839-3854.
Francois, V. and Bier, E. (1995). Xenopus chordin and Drosophila short gastrulation genes encode homologous proteins functioning in dorsal-ventral axis formation. Cell 80, 19-20.
Gallera, J. (1971). Différence de la reactivité à l'inducteur neurogène entre l'ectoblaste de l'aire opaque et celui de l'aire pellucide chez le poulet. Experientia 26, 1953-1954.
Gallera, J. and Ivanov, I. (1964). La compétence neurogéne du feuillet externe du blastoderme de Poulet en fonction du facteur 'temps'. J Embryol Exp Morphol 12, 693.
George-Weinstein, M., Gerhart, J., Reed, R., Flynn, J., Callihan, B., Mattiacci, M., Miehle, C., Foti, G., Lash, J. W. and Weintraub, H. (1996). Skeletal myogenesis: the preferred pathway of chick embryo epiblast cells in vitro. Dev Biol 173, 279-291.
Godsave, S. F. and Slack, J. M. (1989). Clonal analysis of mesoderm induction in Xenopus laevis. Dev Biol 134, 486-490.
Grunz, H. (1992). Suramin changes the fate of Spemann's organizer and prevents neural induction in Xenopus laevis. Mech Dev 38, 133-141.
Grunz, H. and Tacke, L. (1989). Neural differentiation of Xenopus laevis ectoderm takes place after disaggregation and delayed reaggregation without inducer. Cell Differ Dev 28, 211-217.
Gurdon, J. B. (1987). Embryonic induction - molecular prospects. Development 99, 285-306.
Hamburger, V. (1988). The heritage of experimental embryology: Hans Spemann and the Organizer. Oxford: Oxford University Press.
Hammerschmidt, M., Pelegri, F., Mullins, M. C., Kane, D. A., van Eeden, F. J., Granato, M., Brand, M., Furutani-Seiki, M., Haffter, P., Heisenberg, C. P., Jiang, Y. J., Kelsh, R. N., Odenthal, J., Warga, R. M. and Nusslein-Volhard, C. (1996a). dino and mercedes, two genes regulating dorsal development in the zebrafish embryo. Development 123, 95-102.
Hammerschmidt, M., Serbedzija, G. N. and McMahon, A. P. (1996b). Genetic analysis of dorsoventral pattern formation in the zebrafish: requirement of a BMP-like ventralizing activity and its dorsal repressor. Genes Dev 10, 2452-2461.
Hansen, C. S., Marion, C. D., Steele, K., George, S. and Smith, W. C. (1997). Direct neural induction and selective inhibition of mesoderm and epidermis inducers by Xnr3. Development 124, 483-492.
Hardcastle, Z., Chalmers, A. D. and Papalopulu, N. (2000). FGF-8 stimulates neuronal differentiation through FGFR-4a and interferes with mesoderm induction in Xenopus embryos. Curr Biol 10, 1511-1514.
Hawley, S. H., Wunnenberg-Stapleton, K., Hashimoto, C., Laurent, M. N., Watabe, T., Blumberg, B. W. and Cho, K. W. (1995). Disruption of BMP signals in embryonic Xenopus ectoderm leads to direct neural induction. Genes Dev 9, 2923-2935.
Hemmati-Brivanlou, A., Kelly, O. G. and Melton, D. A. (1994). Follistatin, an antagonist of activin, is expressed in the Spemann organizer and displays direct neuralizing activity. Cell 77, 283-295.
Hemmati-Brivanlou, A. and Melton, D. (1997). Vertebrate embryonic cells will become nerve cells unless told otherwise. Cell 88, 13-17.
Hemmati-Brivanlou, A. and Melton, D. A. (1992). A truncated activin receptor inhibits mesoderm induction and formation of axial structures in Xenopus embryos. Nature 359, 609-614.
Hemmati-Brivanlou, A. and Melton, D. A. (1994). Inhibition of activin receptor signaling promotes neuralization in Xenopus. Cell 77, 273-281.
Holley, S. A., Jackson, P. D., Sasai, Y., Lu, B., De Robertis, E. M., Hoffmann, F. M. and Ferguson, E. L. (1995). A conserved system for dorsal-ventral patterning in insects and vertebrates involving sog and chordin. Nature 376, 249-253.
Holowacz, T. and Sokol, S. (1999). FGF is required for posterior neural patterning but not for neural induction. Dev Biol 205, 296-308.
Holtfreter, J. (1933). Eigenschaften und Verbreitung induzierender Stoffe. Naturwissenschaften 21, 766-770.
Holtfreter, J. (1934). Der Einfluss thermischer, mechanischer und chemischer Eingreffe auf die Induzierfaehighkeit von Triton -Keimteilen. Roux' Archiv EntwMech Org. 132, 225-306.
Holtfreter, J. (1945). Neuralization and epidermalization of gastrula ectoderm. J Exp Zool 98, 161-210.
Hongo, I., Kengaku, M. and Okamoto, H. (1999). FGF signaling and the anterior neural induction in Xenopus. Dev Biol 216, 561-581.
Horder, T. J. (2001). The organizer concept and modern embryology: Anglo-American perspectives. Int J Dev Biol 45, 97-132.
Hsu, D. R., Economides, A. N., Wang, X., Eimon, P. M. and Harland, R. M. (1998). The Xenopus dorsalizing factor Gremlin identifies a novel family of secreted proteins that antagonize BMP activities. Mol Cell 1, 673-683.
Hudson, C., Darras, S., Caillol, D., Yasuo, H. and Lemaire, P. (2003). A conserved role for the MEK signalling pathway in neural tissue specification and posteriorisation in the invertebrate chordate, the ascidian Ciona intestinalis. Development 130, 147-159.
Hudson, C. and Lemaire, P. (2001). Induction of anterior neural fates in the ascidian Ciona intestinalis. Mech Dev 100, 189-203.
Hunt, T. E. (1929). Hensen's node as an organizer in the formation of the chick embryo. Anat Rec 42, 22.
Ishimura, A., Maeda, R., Takeda, M., Kikkawa, M., Daar, I. O. and Maeno, M. (2000). Involvement of BMP-4/msx-1 and FGF pathways in neural induction in the Xenopus embryo. Dev Growth Differ 42, 307-316.
Jacobson, M. and Hirose, G. (1981). Clonal organization of the central nervous system of the frog. II. Clones stemming from individual blastomeres of the 32- and 64-cell stages. J Neurosci 1, 271-284.
Jones, C. M., Dale, L., Hogan, B. M., Wright, C. V. E. and Smith, J. C. (1996). Bone Morphogenetic Protein-4 (BMP-4) acts during gastrulation stages to cause ventralization of Xenopus embryos. Development 122, 1545-1554.
Jones, C. M., Lyons, K. M., Lapan, P. M., Wright, C. V. E. and Hogan, B. M. (1992). DVR-4 (Bone Morphogentic Protein-4) as a posterior-ventralizing factor in Xenopus mesoderm induction. Development 115, 639-647.
Kato, Y., Shi, Y. and He, X. (1999). Neuralization of the Xenopus embryo by inhibition of p300/ CREB-binding protein function. J Neurosci 19, 9364-9373.
Kengaku, M. and Okamoto, H. (1995). bFGF as a possible morphogen for the anteroposterior axis of the central nervous system in Xenopus. Development 121, 3121-3130.
Khokha, M. K., Hsu, D., Brunet, L. J., Dionne, M. S. and Harland, R. M. (2003). Gremlin is the BMP antagonist required for maintenance of Shh and Fgf signals during limb patterning. Nat Genet 34, 303-307.
Kim, G. J. and Nishida, H. (2001). Role of the FGF and MEK signaling pathway in the ascidian embryo. Dev Growth Differ 43, 521-533.
Kishimoto, Y., Lee, K. H., Zon, L., Hammerschmidt, M. and Schulte-Merker, S. (1997). The molecular nature of zebrafish swirl: BMP2 function is essential during early dorsoventral patterning. Development 124, 4457-4466.
Klingensmith, J., Ang, S. L., Bachiller, D. and Rossant, J. (1999). Neural induction and patterning in the mouse in the absence of the node and its derivatives. Dev Biol 216, 535-549.
Kroll, K. L. and Amaya, E. (1996). Transgenic Xenopus embryos from sperm nuclear transplantations reveal FGF signaling requirements during gastrulation. Development 122, 3173-3183.
LaBonne, C. and Whitman, M. (1997). Localization of MAP kinase activity in early Xenopus embryos: implications for endogenous FGF signaling. Dev Biol 183, 9-20.
Lamb, T. M. and Harland, R. M. (1995). Fibroblast growth factor is a direct neural inducer, which combined with noggin generates anterior-posterior neural pattern. Development 121, 3627-3636.
Lamb, T. M., Knecht, A. K., Smith, W. C., Stachel, S. E., Economides, A. N., Stahl, N., Yancopolous, G. D. and Harland, R. M. (1993). Neural induction by the secreted polypeptide noggin. Science 262, 713-718.
Launay, C., Fromentoux, V., Shi, D. L. and Boucaut, J. C. (1996). A truncated FGF receptor blocks neural induction by endogenous Xenopus inducers. Development 122, 869-880.
Leclerc, C., Duprat, A. M. and Moreau, M. (1999). Noggin upregulates Fos expression by a calcium-mediated pathway in amphibian embryos. Dev Growth Differ 41, 227-238.
Lerchner, W., Latinkic, B. V., Remacle, J. E., Huylebroeck, D. and Smith, J. C. (2000). Region-specific activation of the Xenopus Brachyury promoter involves active repression in ectoderm and endoderm: a study using transgenic frog embryos. Development 127, 2729-2739.
Lewis, W. H. (1907). Transplantation of the lips of the blastopore in Rana pipiens. Am J Anat 7, 137-141.
Linker, C., De Almeida, I. M. and Stern, C. D. (2004). BMP inhibition is necessary but not sufficient for neural induction. (submitted).
Mangold, O. (1933). Über die Induktionsfähighkeit der verschiedenen Bezirke der Neurula von Urodelen. Naturwissenshaften 21, 761-766.
Mariani, F. V. and Harland, R. M. (1998). XBF-2 is a transcriptional repressor that converts ectoderm into neural tissue. Development 125, 5019-5031.
Matsuo-Takasaki, M., Lim, J. H. and Sato, S. M. (1999). The POU domain gene, XlPOU 2 is an essential downstream determinant of neural induction. Mech Dev 89, 75-85.
McMahon, J. A., Takada, S., Zimmerman, L. B., Fan, C. M., Harland, R. M. and McMahon, A. P. (1998). Noggin-mediated antagonism of BMP signaling is required for growth and patterning of the neural tube and somite. Genes Dev 12, 1438-1452.
Moody, S. A. (1987). Fates of the blastomeres of the 32-cell-stage Xenopus embryo. Dev Biol 122, 300-319.
Moody, S. A. and Kline, M. J. (1990). Segregation of fate during cleavage of frog (Xenopus laevis) blastomeres. Anat Embryol (Berl) 182, 347-362.
Moury, J. D. and Jacobson, A. G. (1989). Neural fold formation at newly created boundaries between neural plate and epidermis in the axolotl. Dev Biol 133, 44-57.
Moury, J. D. and Jacobson, A. G. (1990). The origins of neural crest cells in the axolotl. Dev Biol 141, 243-253.
Nakamura, O. and Toivonen, S. (1978). Organizer: a milestone of a half-century from Spemann, (ed. Amsterdam: Elsevier/North Holland.
Needham, J., Waddington, C. H. and Needham, D. M. (1934). Physico-chemical experiments on the amphibian organizer. Proceedings of the Royal Society of London, ser. B 114, 393-422.
Nieuwkoop, P. D., Botternenbrood, E. C., Kremer, A., Bloesma, F. F. S. N., Hoessels, E. L. M. J., Meyer, G. and Verheyen, F. J. (1952). Activation and organization of the Central Nervous System in Amphibians. Journal of Experimental Zoology 120, 1-108.
Nieuwkoop, P. D. and Nigtevecht, G. V. (1954). Neural activation and transformation in explants of competent ectoderm under the influence of fragments of anterior notochord in urodeles. J Embryol Exp Morphol 2, 175-193.
Oelgeschlager, M., Kuroda, H., Reversade, B. and De Robertis, E. M. (2003). Chordin is required for the Spemann organizer transplantation phenomenon in Xenopus embryos. Dev Cell 4, 219-230.
Oppenheimer, J. M. (1991). Curt Herbst's contributions to the concept of embryonic induction. In A conceptual history of modern embryology., (ed. S. F. Gilbert), pp. 83-90. New York: Plenum Press.
Osada, S., Ohmori, S. Y. and Taira, M. (2003). XMAN1, an inner nuclear membrane protein, antagonizes BMP signaling by interacting with Smad1 in Xenopus embryos. Development 130, 1783-1794.
Otte, A. P., Koster, C. H., Snoek, G. T. and Durston, A. J. (1988). Protein kinase C mediates neural induction in Xenopus laevis. Nature 334, 618-620.
Otte, A. P., Kramer, I. M. and Durston, A. J. (1991). Protein kinase C and regulation of the local competence of Xenopus ectoderm. Science 251, 570-573.
Otte, A. P., Kramer, I. M., Mannesse, M., Lambrechts, C. and Durston, A. J. (1990). Characterization of protein kinase C in early Xenopus embryogenesis. Development 110, 461-470.
Papin, C., van Grunsven, L. A., Verschueren, K., Huylebroeck, D. and Smith, J. C. (2002). Dynamic regulation of Brachyury expression in the amphibian embryo by XSIP1. Mech Dev 111, 37-46.
Pearce, J. J., Penny, G. and Rossant, J. (1999). A mouse cerberus/Dan-related gene family. Dev Biol 209, 98-110.
Peng, Y., Xu, R. H., Mei, J. M., Li, X. P., Yan, D., Kung, H. F. and Phang, J. M. (2002). Neural inhibition by c-Jun as a synergizing factor in bone morphogenetic protein 4 signaling. Neuroscience 109, 657-664.
Pera, E., Ikeda, A., Eivers, E. and De Robertis, E. M. (2003). Integration of IGF, FGF and anti-BMP signals via Smad1 phosphorylation in neural induction. Genes Dev 17, 3023-3028.
Pera, E. M., Wessely, O., Li, S. Y. and De Robertis, E. M. (2001). Neural and head induction by insulin-like growth factor signals. Dev Cell 1, 655-665.
Piccolo, S., Sasai, Y., Lu, B. and De Robertis, E. M. (1996). Dorsoventral Patterning in Xenopus: Inhibition of ventral signals by direct binding of chordin to BMP-4. Cell 86, 589-598.
Pownall, M. E., Welm, B. E., Freeman, K. W., Spencer, D. M., Rosen, J. M. and Isaacs, H. V. (2003). An inducible system for the study of FGF signalling in early amphibian development. Dev Biol 256, 89-99.
Ribisi, S., Jr., Mariani, F. V., Aamar, E., Lamb, T. M., Frank, D. and Harland, R. M. (2000). Ras-mediated FGF signaling is required for the formation of posterior but not anterior neural tissue in Xenopus laevis. Dev Biol 227, 183-196.
Rodriguez-Gallardo, L., Climent, V., Garcia-Martinez, V., Schoenwolf, G. C. and Alvarez, I. S. (1997). Targeted over-expression of FGF in chick embryos induces formation of ectopic neural cells. Int J Dev Biol 41, 715-723.
Rollhauser-ter Horst, J. (1977a). Artificial neural induction in amphibia. I. Sandwich explants. Anat Embryol (Berl) 151, 309-316.
Rollhauser-ter Horst, J. (1977b). Artificial neural induction in amphibia. II. Host embryos. Anat Embryol (Berl) 151, 317-324.
Saint-Jeannet, J. P. and Dawid, I. B. (1994). A fate map for the 32-cell stage of Rana pipiens. Dev Biol 166, 755-762.
Saint-Jeannet, J. P., Huang, S. and Duprat, A. M. (1990). Modulation of neural commitment by changes in target cell contacts in Pleurodeles waltl. Dev Biol 141, 93-103.
Sasai, Y., Lu, B., Piccolo, S. and De Robertis, E. M. (1996). Endoderm induction by the organizer-secreted factors chordin and noggin in Xenopus animal caps. Embo J 15, 4547-4555.
Sasai, Y., Lu, B., Steinbeisser, H. and De Robertis, E. M. (1995). Regulation of neural induction by the Chd and Bmp-4 antagonistic patterning signals in Xenopus. Nature 376, 333-336.
Sasai, Y., Lu, B., Steinbeisser, H., Geissert, D., Gont, L. K. and De Robertis, E. M. (1994). Xenopus chordin: A novel dorsalizing factor activated by Organizer-specific homeobox genes. Cell 79, 779-790.
Sato, S. M. and Sargent, T. D. (1989). Development of neural inducing capacity in dissociated Xenopus embryos. Dev Biol 134, 263-266.
Saxen, L. (1980). Neural induction: past, present, and future. Curr Top Dev Biol 15, 409-418.
Saxén, L. and Toivonen, S. (1962). Primary embryonic induction. London: Academic Press.
Schmidt, J., Francois, V., Bier, E. and Kimelman, D. (1995). Drosophila short gastrulation induces an ectopic axis in Xenopus: evidence for conserved mechanisms of dorsal-ventral patterning. Development 121, 4319-4328.
Schulte-Merker, S., Lee, K. J., McMahon, A. P. and Hammerschmidt, M. (1997). The zebrafish organizer requires chordino. Nature 387, 862-863.
Sheng, G., Dos Reis, M. and Stern, C. D. (2003). Churchill, a zinc finger transcriptional activator, regulates the transition from gastrulation to neurulation. Cell 115, 603-613.
Smith, W. C. and Harland, R. M. (1992). Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell 70, 829-840.
Smith, W. C., Knecht, A. K., Wu, M. and Harland, R. M. (1993). Secreted noggin protein mimics the Spemann organizer in dorsalizing Xenopus mesoderm. Nature 361, 547-549.
Sokol, S. Y., Klingensmith, J., Perrimon, N. and Itoh, K. (1995). Dorsalizing and neuralizing properties of Xdsh, a maternally expressed Xenopus homolog of dishevelled. Development 121, 1637-1647.
Spemann, H. (1921). Die Erzeugung tierischer Chimären durch heteroplastische Transplantation zwischen Triton cristatus und taeniatus. W Roux Arch EntwMech Org 48, 533-570.
Spemann, H. (1938). Embryonic Development and Induction: Yale University Press.
Spemann, H. and Mangold, H. (1924). Uber induktion von Embryonalanlagen durch implantation artfremder Organisatoren. Roux Arch. EntwMech. Org. 100, 599-638.
Stern, C. D. (2001). Initial patterning of the central nervous system: how many organizers? Nat Rev Neurosci 2, 92-98.
Storey, K. G., Crossley, J. M., De Robertis, E. M., Norris, W. E. and Stern, C. D. (1992). Neural induction and regionalisation in the chick embryo. Development 114, 729-741.
Storey, K. G., Goriely, A., Sargent, C. M., Brown, J. M., Burns, H. D., Abud, H. M. and Heath, J. K. (1998). Early posterior neural tissue is induced by FGF in the chick embryo. Development 125, 473-484.
Streit, A., Berliner, A. J., Papanayotou, C., Sirulnik, A. and Stern, C. D. (2000). Initiation of neural induction by FGF signalling before gastrulation. Nature 406, 74-78.
Streit, A., Lee, K. J., Woo, I., Roberts, C., Jessell, T. M. and Stern, C. D. (1998). Chordin regulates primitive streak development and the stability of induced neural cells, but is not sufficient for neural induction in the chick embryo. Development 125, 507-519.
Streit, A., Sockanathan, S., Perez, L., Rex, M., Scotting, P. J., Sharpe, P. T., Lovell-Badge, R. and Stern, C. D. (1997). Preventing the loss of competence for neural induction: HGF/SF, L5 and Sox-2. Development 124, 1191-1202.
Streit, A. and Stern, C. D. (1999a). Establishment and maintenance of the border of the neural plate in the chick: involvement of FGF and BMP activity. Mech Dev 82, 51-66.
Streit, A. and Stern, C. D. (1999b). Mesoderm patterning and somite formation during node regression: differential effects of chordin and noggin. Mech Dev 85, 85-96.
Strong, C. F., Barnett, M. W., Hartman, D., Jones, E. A. and Stott, D. (2000). Xbra3 induces mesoderm and neural tissue in Xenopus laevis. Dev Biol 222, 405-419.
Sullivan, S. A., Akers, L. and Moody, S. A. (2001). foxD5a, a Xenopus winged helix gene, maintains an immature neural ectoderm via transcriptional repression that is dependent on the C-terminal domain. Dev Biol 232, 439-457.
Suzuki, A., Chang, C., Yingling, J. M., Wang, X. F. and Hemmati-Brivanlou, A. (1997a). Smad5 induces ventral fates in Xenopus embryo. Dev Biol 184, 402-405.
Suzuki, A., Ueno, N. and Hemmati-Brivanlou, A. (1997b). Xenopus msx1 mediates epidermal induction and neural inhibition by BMP4. Development 124, 3037-3044.
Tam, P. P. and Steiner, K. A. (1999). Anterior patterning by synergistic activity of the early gastrula organizer and the anterior germ layer tissues of the mouse embryo. Development 126, 5171-5179.
Thomas, P. and Beddington, R. (1996). Anterior primitive endoderm may be responsible for patterning the anterior neural plate in the mouse embryo. Curr Biol 6, 1487-1496.
Toivonen, S. (1938). Spezifische Induktionsleistungen von abnormen Induktoren im Implantatversuch. Ann. Soc. zool.-bot. fenn. Vanamo 6, 1-12.
Toivonen, S. (1940). Über die Leistungsspezifität der abnormen Induktoren im Implantatversuch bei Triton. Ann. Acad. Sci. fenn., ser. A., IV 55, 1-150.
Toivonen, S., Tarin, D., Saxen, L., Tarin, P. J. and Wartiovaara, J. (1975). Transfilter studies on neural induction in the newt. Differentiation 4, 1-7.
Tonegawa, A., Funayama, N., Ueno, N. and Takahashi, Y. (1997). Mesodermal subdivision along the mediolateral axis in chicken controlled by different concentrations of BMP-4. Development 124, 1975-1984.
Tonegawa, A. and Takahashi, Y. (1998). Somitogenesis controlled by Noggin. Dev Biol 202, 172-182.
Tsuda, H., Sasai, N., Matsuo-Takasaki, M., Sakuragi, M., Murakami, Y. and Sasai, Y. (2002). Dorsalization of the neural tube by Xenopus tiarin, a novel patterning factor secreted by the flanking nonneural head ectoderm. Neuron 33, 515-528.
Uchikawa, M., Ishida, Y., Takemoto, T., Kamachi, Y. and Kondoh, H. (2003). Functional analysis of chicken Sox2 enhancers highlights an array of diverse regulatory elements that are conserved in mammals. Dev Cell 4, 509-519.
Umbhauer, M., Penzo-Mendez, A., Clavilier, L., Boucaut, J. and Riou, J. (2000). Signaling specificities of fibroblast growth factor receptors in early Xenopus embryo. J Cell Sci 113, 2865-2875.
Verschueren, K., Remacle, J. E., Collart, C., Kraft, H., Baker, B. S., Tylzanowski, P., Nelles, L., Wuytens, G., Su, M. T., Bodmer, R., Smith, J. C. and Huylebroeck, D. (1999). SIP1, a novel zinc finger/homeodomain repressor, interacts with Smad proteins and binds to 5'-CACCT sequences in candidate target genes. J Biol Chem 274, 20489-20498.
von Baer, K. E. (1828). Über Entwickelungsgeschichte der Thiere. Beobachtung und Reflexion. Königsberg: Bornträger.
Waddington, C. H. (1930). Developmental mechanics of chick and duck embryos. Nature 125, 924- 925.
Waddington, C. H. (1932). Experiments on the development of chick and duck embryos cultivated in vitro. Phil. Trans. Roy. Soc. Lond. B 221, 179-230.
Waddington, C. H. (1933a). Induction by coagulated organisers in the chick embryo. Nature (London) 131, 275.
Waddington, C. H. (1933b). Induction by the Primitive Streak and its Derivatives in the Chick. Journal of Experimental Biology 10, 38-48.
Waddington, C. H. (1934). Experiments on embryonic induction. J. exp. Biol. 11, 211-227.
Waddington, C. H. (1936). Organizers in Mammalian Development. Nature 138, 125.
Waddington, C. H. (1937). Experiments on determination in the rabbit embryo. Arch Biol 48, 273-290.
Waddington, C. H. (1940). Organizers and Genes. London: Cambridge University Press.
Wagner, D. S. and Mullins, M. C. (2002). Modulation of BMP activity in dorsal-ventral pattern formation by the chordin and ogon antagonists. Dev Biol 245, 109-123.
Wessely, O., Agius, E., Oelgeschlager, M., Pera, E. M. and De Robertis, E. M. (2001). Neural induction in the absence of mesoderm: beta-catenin-dependent expression of secreted BMP antagonists at the blastula stage in Xenopus. Dev Biol 234, 161-173.
Wilson, P. A. and Hemmati-Brivanlou, A. (1995). Induction of epidermis and inhibition of neural fate by Bmp-4. Nature 376, 331-333.
Wilson, P. A., Lagna, G., Suzuki, A. and Hemmati-Brivanlou, A. (1997). Concentration-dependent patterning of the Xenopus ectoderm by BMP4 and its signal transducer Smad1. Development 124, 3177-3184.
Wilson, S. I. and Edlund, T. (2001). Neural induction: toward a unifying mechanism. Nat Neurosci 4, 1161-1168.
Wilson, S. I., Graziano, E., Harland, R., Jessell, T. M. and Edlund, T. (2000). An early requirement for FGF signalling in the acquisition of neural cell fate in the chick embryo. Curr Biol 10, 421-429.
Wilson, S. I., Rydstrom, A., Trimborn, T., Willert, K., Nusse, R., Jessell, T. M. and Edlund, T. (2001). The status of Wnt signalling regulates neural and epidermal fates in the chick embryo. Nature 411, 325-330.
Winnier, G., Blessing, M., Labosky, P. A. and Hogan, B. M. (1995). Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev 9, 2105-2116.
Witta, S. E., Agarwal, V. R. and Sato, S. M. (1995). XIPOU 2, a noggin-inducible gene, has direct neuralizing activity. Development 121, 721-730.
Xu, R. H., Kim, J., Taira, M., Lin, J. J., Zhang, C. H., Sredni, D., Evans, T. and Kung, H. F. (1997). Differential regulation of neurogenesis by the two Xenopus GATA-1 genes. Mol Cell Biol 17, 436-443.
Xu, R. H., Kim, J., Taira, M., Zhan, S., Sredni, D. and Kung, H. F. (1995). A dominant negative bone morphogenetic protein 4 receptor causes neuralization in Xenopus ectoderm. Biochem Biophys Res Commun 212, 212-219.
Yabe, S., Tanegashima, K., Haramoto, Y., Takahashi, S., Fujii, T., Kozuma, S., Taketani, Y. and Asashima, M. (2003a). FRL-1, a member of the EGF-CFC family, is essential for neural differentiation in Xenopus early development. Development 130, 2071-2081.
Yabe, T., Shimizu, T., Muraoka, O., Bae, Y. K., Hirata, T., Nojima, H., Kawakami, A., Hirano, T. and Hibi, M. (2003b). Ogon/Secreted Frizzled functions as a negative feedback regulator of Bmp signaling. Development 130, 2705-2716.
Zhang, H. and Bradley, A. (1996). Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development 122, 2977-2986.
Zimmerman, L. B., De Jesus-Escobar, J. M. and Harland, R. M. (1996). The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell 86, 599-606.
Figure legends
Fig. 1: Diagram of the "organizer graft" experiment of Spemann & Mangold (1924). The dorsal lip (red) of the blastopore (thick black line) of a donor newt at mid-gastrula stage is transplanted to the opposite (ventral) side of a host.
Fig. 2: Diagrams bu Hilde Mangold, illustrating the results of her organizer graft experiments (Fig. 1). The upper four figures are Indian ink drawings prepared as for publication, showing her embryos (Um25b, Um27a and two views of Um16) in whole mount. The lower part of the figure shows sketches from the sections of her most famous grafted embryo (Um132) where the donor tissue is colored red. Note that the mesoderm including somite and notochord is derived from the donor, while the adjacent nervous system is not. Access to the notebook and permission to reproduce them by courtesy of Jenny Narraway and the Embryological Collection of the Hubrecht Laboratory, Utrecht.
Fig. 3: The "default model" in Xenopus. On the left is a rough fate map of a blastula-stage embryo (organizer in red, ventral mesoderm in pink, neural in blue, epidermis yellow, yolky endoderm green). The inhibitory arrows represent BMP antagonist activity emanating from the organizer. On the right is a "genetic" diagram of the inductive interactions proposed by the model: ectoderm cells (represented by the grey boxes) have an autonomous tendency to differentiate into neural tissue, but are prevented from doing this and directed instead to epidermis by BMP4, which is expressed ubiquitously. Near the organizer, BMP antagonists block BMP4 signaling allowing neighboring ectoderm cells to develop according to their "default" neural fate.
Fig. 4: Summary of the main experiments supporting the "default model" (Fig. 3) as done in Xenopus. The leftmost two columns illustrate the results of cell dissociation experiments, the next two show the effects of incubating animal caps with BMP antagonists, and the last two columns summarize the most common type of "animal cap" experiment. The lower box shows the usual results of these experiments where + implies expression of markers for the tissue shown, - means no expression, and up- or down-arrows represent up- or down-regulation respectively.
Fig. 5: Organizer graft experiment in the chick, also demonstrating the changes in inducing ability of the organizer with increasing age of the donor. The middle diagram shows a host chick embryo, at stage 4. This embryo simultaneously receives a graft of a quail stage 4 node on its left and a quail stage 6 node on its right. The lower panel shows the result of this experiment, after in situ hybridization (purple) for the hindbrain marker Krox-20 (expressed in rhombomeres 3 and 5, arrows) and staining with an anti-quail antibody (brown). The young graft has induced a complete axis including the entire head, while the older graft on the right has generated a short axis, mostly derived from the graft itself and lacking rostral structures including the Krox-20-expressing region. Experiment performed by Kate Storey (Storey et al. 1992).
Fig. 6: Results of chick misexpression experiments. The upper diagram shows the two main types of misexpression experiments usually done in whole chick embryos: a graft of cultured COS cells that had been transfected with an expression plasmid encoding a secreted factor (left), and in vivo electroporation of an expression plasmid encoding the desired protein (which may be a transcription factor, secreted protein or any other construct) directly into a test region of the epiblast. The lower table summarizes the results of the main experiments done in whole chick embryos. + indicates induction, - no induction, n.e. no effect. In the first three examples (node, hypoblast, FGF8) the time (hours) of exposure required to obtain induction of the marker is shown.
Brachyury (Bra) is a marker for mesoderm. Sox2 is the "definitive" neural plate marker and the remaining markers (ERNI, Otx2, Sox3, ChCh) are expressed in the early epiblast including the prospective neural territory but do not indicate commitment of the cells to a neural fate. "αWnt" is a mixture of three different Wnt antagonists (crescent, NFz8 and Dkk1) and a multifunctional antagonist of Wnt, BMP and Nodal (Cerberus). Note that no combination of factors can mimic the induction of Sox2 by the node.
† Chordin induces an ectopic primitive streak when misexpressed inside the embryo (Streit et al., 1999b) but does not induce neural markers in competent epiblast.
* BMP4 misexpressed by electroporation within the neural plate inhibits Sox2 but not Sox3. When using COS cells there is no effect.
Based on experiments by Streit et al. (1999a, b, 2000) and C. Linker, I. de Almeida and C. D. Stern (unpublished).
Fig. 7: Model summarizing the regulation and functions of ChCh during early development. A-D: the embryologist's view; E: the geneticist's view. In A-D, embryos are shown at four stages, with their germ layers exploded. A. At stages XI-XII, the hypoblast (brown) emits FGF8 which induces the early pre-neural genes ERNI and Sox3 (orange) in the overlying epiblast (yellow), but the cells in this domain are still uncommitted. At this stage Nodal is expressed in the posterior (right) epiblast but is inhibited by Cerberus secreted by the hypoblast. B. At stages XIII-2, the hypoblast is displaced from the posterior part of the embryo by the endoblast (white) which allows Nodal signaling, in synergy with FGF, to induce Brachyury and Tbx6L and ingression (red arrows) to form the primitive streak (red). C. At stages 3+-4, continued FGF signaling now induces Churchill in a domain of the epiblast (turquoise). The border of the epiblast territory destined to ingress to form mesoderm is shown with a dashed black line. D. At the end of stage 4, Churchill induces Sip1, which blocks Brachyury, Tbx6L and further ingression of epiblast into the streak. The epiblast remaining outside the streak (blue) is now sensitized to neural inducing signals emanating from the node (blue arrows). E. The same model shown as a genetic cascade. Interactions described in this paper are shown as black lines, those from the literature are faint. The time axis runs vertically, wherein the color gradients indicate progressive commitment to epidermis (yellow), neural (blue) and mesoderm (red). BMP/Smad/Sip1 interactions regulate the epidermis-neural plate border, while ChCh/Sip1/FGF/Bra/Tbx6 regulate the mesoderm-neural decision. Reproduced from Sheng et al. (2003) with permission of Cell Press.
 Close
Close

