New publication in Nature by Hadjivasiliou lab
22 December 2021
Morphogen gradient scaling by recycling of intracellular Dpp
Multicellular life encompasses an extraordinary diversity in size across evolution. Still, the same molecules and developmental programs are used repeatedly by evolution to mould size, shape, and morphological patterns across length scales. A similar transformation in size occurs within species during development. Indeed, from invertebrates to mammals, multicellular organisms grow from a single cell to form an adult body with stereotyped size, shape, and morphological structures. This means that the machineries that control size and patterning during development must adapt to organism size so that they can operate at these changing length scales. Can the processes through which developmental mechanisms adapt, or scale, to size during development also help shed light on how the same mechanism transcend size across evolution?
In this study we explore how morphogen gradients form and scale to organ size. Morphogens are highly conserved across species, and they orchestrate both patterning and growth throughout development. They are secreted molecules that form spatial concentration gradients and patterns emerge when cells activate target genes as a function of the morphogen concentration they sense. So, the gradient shape often specifies the morphological pattern of a growing tissue or organism. At the same time, morphogens dictate cell proliferation and so their signalling dynamics can impact both the tempo of development and the final organ or animal size. Morphogen scaling has been documented in a plethora of model systems and implies that the gradient range expands as the tissue grows to remain proportional to the tissue size. This ensures that patterns remain proportionate to size during developmental growth and across adults that vary in size. But how is this scaling achieved?
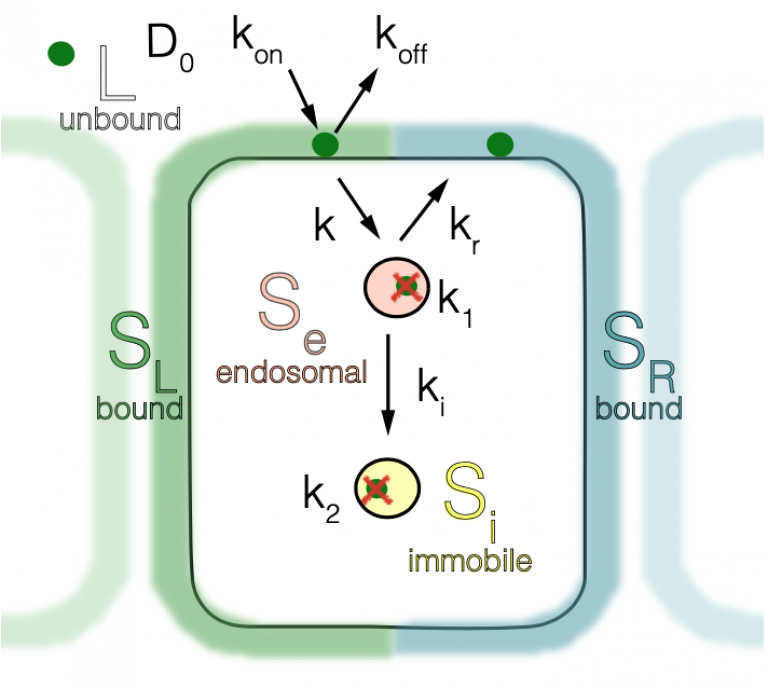
To discover the mechanism through which morphogens are transported in tissues and scale to tissue size we used theoretical modelling and experiments in the Drosophila melanogaster wing. The theoretical description we employed incorporates all key processes that could in principle contribute to morphogen gradient formation, from extracellular diffusion, binding and unbinding to the cell membrane, internalization of molecules, recycling of molecules that were already endocytosed and degradation. We described these processes using reaction diffusion equations that were used alongside several experimental assays to estimate all the trafficking rates that contribute to Dpp gradient formation. This allowed us to establish what are the key processes that underlie Dpp gradient formation. Whereas in small tissues early in development the Dpp gradient is short and is formed mainly through extracellular diffusion and irreversible capture by receptors, as the tissue grows molecules that were already internalized return to the extracellular space and continue to spread in the tissue. This increased contribution of recycled molecules as the tissue grows is what allows the gradient of Dpp to scale to the tissue size.
Besides discovering the process through which the Dpp gradient forms and scales, this study highlights how versatile developmental machineries can be. It is not the properties of the morphogen molecules per se that determine the length scale of the morphogen gradient but rather the rates at which molecules travel in and out of cells and across the tissue. Since these processes are genetically encoded, evolution can modulate length- and timescales of pattern formation and growth across orders of magnitude using the same molecules and mechanisms.
Links
- Full paper in Nature
- Dr. Zena Hadjivasiliou’s academic profile
- Francis Crick Institute
- UCL Physics & Astronomy
- UCL Mathematical & Physical Sciences
- UCL Institute for the Physics of Living Systems
Image
Scheme of 5 pools and 8 transport rates of morphogen transport.
 Close
Close

