Transgenic mice in the study of development (Dr Hazel Smith)
Gastrulation in the mouse takes place shortly after implantation and is essentially
a similar process to gastrulation in other
vertebrates such as Xenopus. Although the
topography is very different the same genetic and cell signalling pathways
appear to be involved.
Mouse
development from implantation to mid-gastrulation
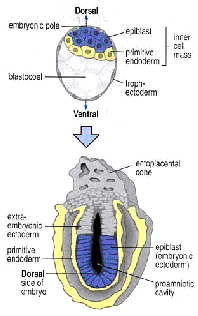 Implantation
takes place between E4.5 and E6. Visceral endoderm cells (future amnion,
yellow) migrate so that they come to line the entire blastocoel.
Trophectoderm (grey) cells adjacent to the ICM
proliferate to form the ectoplacental cone and extraembryonic ectoderm. By E6 the epiblast (future embryo, blue) still forms a single layer
but has become cup shaped. The whole structure is now termed the egg
cylinder.
Implantation
takes place between E4.5 and E6. Visceral endoderm cells (future amnion,
yellow) migrate so that they come to line the entire blastocoel.
Trophectoderm (grey) cells adjacent to the ICM
proliferate to form the ectoplacental cone and extraembryonic ectoderm. By E6 the epiblast (future embryo, blue) still forms a single layer
but has become cup shaped. The whole structure is now termed the egg
cylinder.
Gastrulation begins between E6 and E6.5 with the formation of the primitive streak
at the posrterior pole of the epiblast.
Epiblast cells that move throught
the streak give rise to mesoderm as in Xenopus.
Between E6.5 and E7.5 the streak elongates to the tip of the cylinder and by
E8.5 organogenesis has begun with anterior structures such as heart and brain
being the first to develop. At the anterior end of the streak is a
specialised structure called the node, which is equivalent to the frog dorsal blastopore lip or organiser.
Transplantation of the node has the same effect as that of the organiser
(induction of a secondary axis) and both node ands organiser express similar
sets of transcription factors and signalling molecules (see below).
Around mid-gastrulation the mouse embryo can be
imagined as as being a Xenopus
embryo which has been split open just anterior to the dorsal blastopore lip and turned inside out (see diagram).

Gene
function in mouse and Xenopus
development
I. Brachyury function in mesoderm development
Methods for
studying development in mouse complement those in Xenopus.
In Xenopus genes are usually identified by
having particular expression patterns or homology to developmental regulators
in other systems. Their function can then be studied by analysing the
effects of overexpression (gain of function), by
injecting mRNA etc. In the mouse genes identified by expression or
homology (see above) can be studied by analysing the phenotypic effects of
targeted mutations (loss of function). In some cases genes may also be
identified (as in Drosophila ) by the phenotype
of mutant animals. Mutations can be spontaneous (e.g. Brachyury-T)
or insertional (e.g. in Nodal).
The Xenopus gene Xbra
is expressed transiently in presumptive mesoderm and later in the
notochord. It is one of the first genes to be switched on when mesoderm
inducing factors are added to presumptive ectoderm and injection of Xbra mRNA itself causes presumptive ectoderm to
differentiate as mesoderm. This suggests but does not prove that Xbra
is normally required for or directs mesoderm development. The genes Brachyury and Tbx6 are mouse homologues of Xbra. Like Xbra,
Brachyury , is expressed in presumptive mesoderm as it passes through
the streak and in notochord. Tbx6 is expressed in mesoderm
slightly later than Brachyury and is absent
from notochord. Spontaneous mutations in Brachyury
cause tail defects in heterozygotes. Homozygotes lack notochord and trunk mesoderm. This
confirms that Brachyury is required for
mesoderm formation but, due to the lack of the
notochord, the mutants are too disorganised to tell what has happened to the
cells that would have made the trunk mesoderm - do they just fail to
differentiate or do they adopt an alternative fate? Knockouts of Tbx6
have been made using ES cells. In homozygous mutant for Tbx6
mesoderm is replaced by ectopic neural tubes
supporting the later alternative.
Organising
the anterior-posterior pattern
Organiser/node
grafts can induce the formation of a secondary axis in Xenopus
and mice suggesting that signals from the organiser confer anterior-posterior
pattern on both mesoderm and ectoderm. Although early grafts in Xenopus induce the formation of a complete secondary
axis including the head later grafts only induce trunk structures. This
has been interpreted as meaning that separate signalling pathways organise the
head and the trunk. Overexpression of genes
expressed in the organiser to see if this can induce trunk or head formation
have been used to try to identify these pathways. Noggin and chordin (BMP inhibitors) induce trunk duplications, while
nodal related genes induce complete axes and another gene cerebus
induces only head formation. Cerebus is
in fact not expressed in the node proper but just anterior to it. The
equivalent region of the mouse embryo is the anterior visceral endoderm (AVE)
and in the mouse is physically separated from the node. Could contact with this
region induce head identity before cells pass through the node?
A number of
genes (nodal, otx2, hex1 and GATA4) are expressed
in AVE before the node forms but later come to be expressed in the node.
Knockouts of otx2, hex1 and GATA4
and an insertional mutant in Nodal
all have very severe effects on head and mesoderm development but this
could be due to their expression in the node. However because the AVE is extraembryonic it is possible to separate the AVE and node
specific effects of these genes by exploiting the fact that injected ES cells
will only contribute to embryonic tissue. This means that if :-
1) 1) mutant ES cells are injected into WT blastocysts
the AVE will be wild type and the embryo at least partly mutant.
2) 2) Wild type
ES cells are injected into mutant blastocysts the AVE
will be completely mutant and the embryo at least partly wild type.
This type
of experiment has been done for Nodal and the results show that head
formation can occur normally as long as the AVE is wild type confirming that
the AVE and not just the node is important for antero-posterior
patterning.