Amino acids as the source of ammonium ions
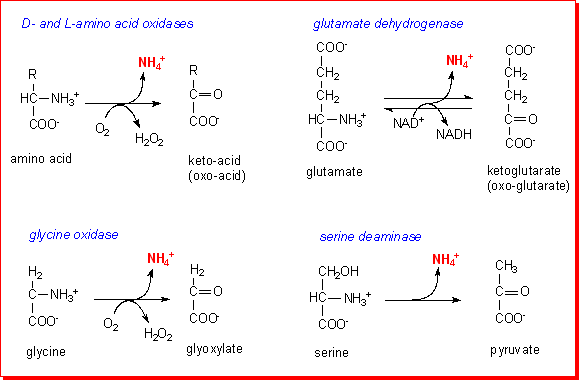 There
is a general L-amino acid oxidase that catalyses the oxidative deamination of
more or less all amino acids, but this has low activity and is unimportant in
terms of amino acid metabolism. D-Amino acid oxidase has a relatively high activity
in the kidney, but this is important for detoxication of the small amounts of
D-amino acids that come from bacteria and a few other sources, not general amino
acid metabolism.
There
is a general L-amino acid oxidase that catalyses the oxidative deamination of
more or less all amino acids, but this has low activity and is unimportant in
terms of amino acid metabolism. D-Amino acid oxidase has a relatively high activity
in the kidney, but this is important for detoxication of the small amounts of
D-amino acids that come from bacteria and a few other sources, not general amino
acid metabolism.
Three amino acids can be deaminated directly: glutamate (catalysed by glutamate
dehydrogenase), glycine (catalysed by glycine oxidase) and serine (catalysed
by serine dehydrogenase).
Two of these enzymes, glutamate dehydrogenase and glycine oxidase, are especially
important, since the keto-acid products (ketoglutarate and glyoxylate respectively)
can readily be reaminated to the parent amino acid by transfer of the amino
acid from a donor amino acid - the process of transamination.
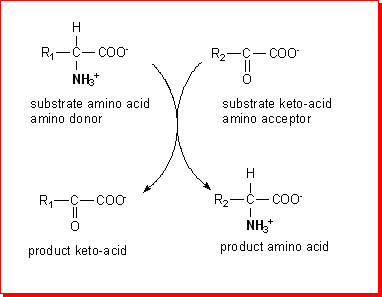
All of the amino acids apart from lysine undergo transamination; some transaminases
use ketoglutarate as the acceptor keto-acid, forming glutamate which is then
a substrate for glutamate dehydrogenase. Many others use oxaloacetate as the
acceptor keto-acid, forming aspartate, which then transfers its amino group
to ketoglutarate. Thus, by linking a series of transaminases and glutamate dehydrogenase
there is a pathway for the overall deamination of most amino acids.
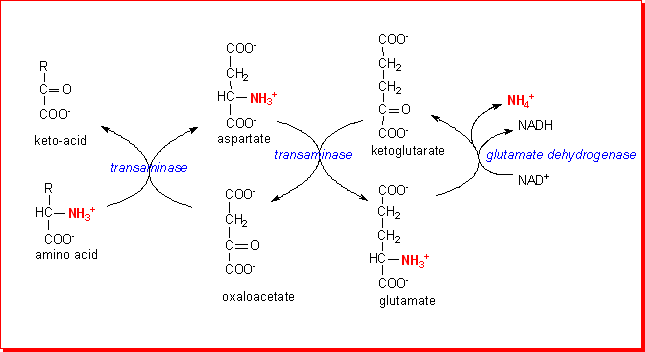
Many transaminases use pyruvate as the acceptor keto-acid, forming alanine,
and there is an active alanine-glyoxylate transaminase in peroxisomes that transfers
the amino group onto glyoxylate, forming glycine, which is then a substrate
for glycine oxidase. This provides another pathway for the overall deamination
of a variety of amino acids.

Genetic defects in alanine-glyoxylate transaminase (either low activity or,
rarely, a mutation that leads to the enzyme being in mitochondria rather than
peroxisomes) results in hyperoxaluria. The glyoxylate formed by glycine oxidase
cannot be recycled to glycine by transamination, but accumulates, and is a substrate
for oxidation catalysed by lactate dehydrogenase, forming oxalate. Oxalate crystallises
in the liver and kidneys, leading, in severe cases, to early death.
 There
is a general L-amino acid oxidase that catalyses the oxidative deamination of
more or less all amino acids, but this has low activity and is unimportant in
terms of amino acid metabolism. D-Amino acid oxidase has a relatively high activity
in the kidney, but this is important for detoxication of the small amounts of
D-amino acids that come from bacteria and a few other sources, not general amino
acid metabolism.
There
is a general L-amino acid oxidase that catalyses the oxidative deamination of
more or less all amino acids, but this has low activity and is unimportant in
terms of amino acid metabolism. D-Amino acid oxidase has a relatively high activity
in the kidney, but this is important for detoxication of the small amounts of
D-amino acids that come from bacteria and a few other sources, not general amino
acid metabolism.

