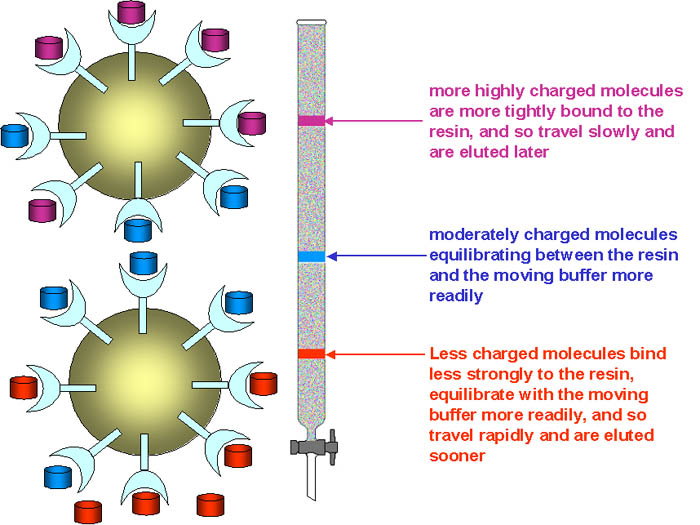
Different proteins have different numbers of acidic (aspartate and glutamate) and basic (arginine, histidine and lysine) amino acid side chains exposed on the surface, and therefore at a given pH they will have different nett charges. This can be exploited as a means of separating proteins by use of chromatography on a column of an ion exchange resin. More highly charged proteins will bind to the resin more strongly, and hence be retarded in their passage through the column. Commonly, proteins are eluted from the column by either:

The resin used may be either a cation or an anion exchanger; those commonly used in protein purification include:
| functional group | support medium | |
| weak anion exchangers | ||
| DEAE-Sephacel | diethylethylaminoethyl | Sephacel |
| DEAE-Sephadex | diethylethylaminoethyl | Sephadex |
| PEI-cellulose | polyethyleneimine | cellulose |
| weak cation exchangers | ||
| CM-Sephacel | carboxymethyl | Sephacel |
| CM-Sephadex | carboxymethyl | Sephadex |
| Bio-Rex 70 | carboxylic acid | acrylic polymer |
In this simulation you will attempt to separate the proteins by anion exchange chromatography on DEAE-Sephadex, using a sodium chloride gradient for elution. Your first task is to decide an appropriate pH for the initial buffer. This is determined by experiment - if you choose a pH at which the enzyme elutes in the void volume of the column (i.e.it has not bound to the resin) then you should choose a higher pH, in order to ensure that it has a sufficient positive charge to bind to the reactive groups on the resin.