Proteins are soluble in aqueous media because they have hydrophilic amino acid side-chains facing outwards that can interact with water. These are provided by the basic amino acids (arginine, histidine, arginine and lysine), the acidic amino acids (aspartate and glutamate) and the neutral hydrophilic amino acids (asparagine, glutamine, serine, threonine, tyrosine and cysteine).
Any compound that interferes with these interactions between amino acid side-chains and water, by reducing the available water, will reduce the solubility of the protein. As interactions with water become less marked, so protein-protein interactions become more important, and the protein will aggregate and come out of solution. Provided that the temperature is maintained low enough (around 4C), the protein is not irreversibly denatured, but the precipitate can be redissolved in buffer.
A number of different methods can be used to reduce hydrophilic interactions and precipitate out proteins reasonably selectively, including:
Ammonium sulphate is highly hydrated, and a concentrated ammonium sulphate
solution reduces the available water very considerably.
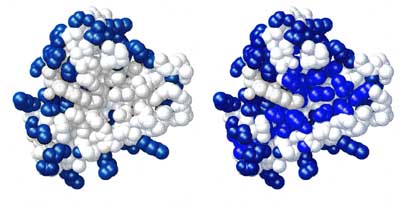
The diagram on the right shows two proteins, with their hydrophilic regions coloured blue.The protein on the left has relatively few hydrophilic regions, and hence will aggregate and precipitate at a relatively low concentration of ammonium sulphate - perhaps around 20 - 30% saturation. By contrast, the protein on the right has considerably more hydrophilic regions, and hence will remain in solution until the concentration of ammonium sulphate is considerably higher - perhaps around 50 - 60% saturation.
This means that it is possible to separate proteins from a mixture on the basis of their relative hydrophilicity by gradually increasing the concentration of ammonium sulphate.
At each stage you calculate the volume of saturated ammonium sulphate solution that will be required to achieve a given percentage saturation of your enzyme preparation, which is typically a crude tissue homogenate or perhaps a high-speed supernatant of a tissue homogenate. The ice-cold saturated solution of ammonium sulphate is added slowly to the protein solution, in an ice bath, and stirred continually. When the required amount has been added, the solution is centrifuged, and the precipitate collected, and redissolved in buffer. A higher degree of saturation with ammonium sulphate is then achieved by adding more saturated ammonium sulphate solution in the same way.
Initially you would probably use a number of wide ranges of ammonium sulphate saturation, say 0 - 50% and see whether or not your enzyme is precipitated. If it is, then you can refine the range until you achieve maximum recovery of the enzyme and maximum removal of interfering proteins.
Removing the ammonium sulphate by dialysis
Having precipitated a protein fraction that contains most of your enzyme, and
redissolved it in buffer, it is necessary to remove the ammonium sulphate before
you can proceed to subsequent steps in the purification process. The simplest
way to achieve this is to dialyse the solution. 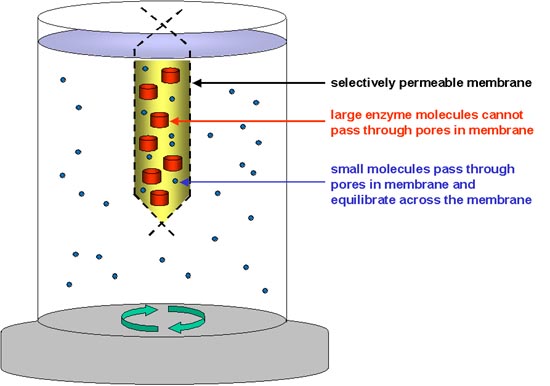
As shown in the diagram, the enzyme solution is placed in a bag of selectively permeable membrane (e.g. cellophane), immersed in a large volume of buffer that is stirred and maintained at about 4C.The membrane has pores that will permit small molecules such as ammonium and sulphate ions to cross, and hence equilibrate in the larger volume of buffer outside, while not permitting large protein molecules to cross. If the buffer is changed several times, allowing several hours each time for the ammonium sulphate to equilibrate, more or less all of it will be removed from the protein solution.
Dialysis will increase the volume of the enzyme solution, because of the initial osmotic effect of the ammonium sulphate; (this is why it is important to leave an air gap at the top of the membrane tube, to prevent it bursting).
Removing the ammonium sulphate by gel filtration
An alternative way of removing the ammonium sulphate is by gel filtration, using e.g. Sephadex G25, which has small pores that will retard small molecules such as ammonium and sulphate ions, but will exclude large protein molecules, so that they are eluted in the void volume of the column. This means that the early eluate will contain the proteins, more or less free from ammonium sulphate.
