Preclinical
Overview: Fetal growth restriction (FGR) is a pregnancy complication in which the baby in the womb (fetus) does not grow as well as it should. In some pregnancies the fetus stops growing very early in pregnancy. FGR is associated with stillbirth and neonatal death, and survivors have a high risk of cardiovascular disease and diabetes. There is no treatment that can improve fetal growth. The underlying problem is poor blood flow in the mother's uterine arteries. We have developed a novel therapy for FGR. This delivers a short term gene therapy (adenovirus vector, Ad) containing the Vascular Endothelial Growth Factor (VEGF) gene to the maternal uterine arteries. We have shown that this maternal VEGF growth factor gene therapy improves uterine artery blood flow, and improves fetal growth.
Design: Preclinical studies are being done in growth restricted guinea pigs that have been born after our treatment. We are studying their blood pressure, sugar control and their development.
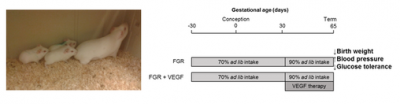
Experimental timeline for follow-up study on Guinea Pig pups
Clinical
EVERREST Clinical Trial: The EVERREST Project aims to carry out a phase I/IIa trial of maternal VEGF growth factor gene therapy for severe early onset fetal growth restriction (FGR). Reproductive toxicology work will complete in August 2016. We will apply for ethical and regulatory approval at the end of 2016, aiming to start the trial in 2017. This is potentially the first trial of gene therapy in pregnancy. An important part of the project conducted with Queen Mary University of London considered the ethical implications of the proposed intervention.
EVERREST Prospective Study: Along with our clinical partners in Germany, Spain, and Sweden we are studying in detail pregnancies affected by severe early onset FGR. We are collecting clinical data, including serial ultrasound scans and neuro-developmental follow-up for surviving babies, along with a bio-bank of samples taken at enrolment and delivery. The findings of this study will help to interpret the safety data from the EVERREST Clinical Trial. We are also investigating ultrasound and biochemical predictors of outcome for affected pregnancies. We are working with our partners Magnus Growth, FinVector, University of Eastern Finland and Euram to translate the therapy into the clinic.
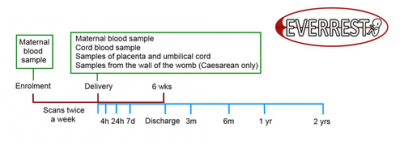
Participant timeline in the EVERREST Prospective Study
Team (Preclinical): Dr Carlo Rossi, Dr Owen Vaughan
Team (Clinical): Dr Rebecca Spencer, Susan Tebbs, Jade Dyer, Alison Evans
Funding: Action Medical Research, EVERREST FP7, Magnus Life Science

 Close
Close







