Prenatal Cell and Gene Therapy
Professor Anna David leads a team developing prenatal treatment of severe early onset and life-threatening disorders using gene and cellular therapy, and to investigate the efficacy, safety and ethical issues of such treatment.
Background
Research into fetal gene therapy began under a 5 year MRC Programme grant (Professor Charles Rodeck) in collaboration with the Gene Therapy Research Group (Professor Charles Coutelle) at Imperial College, London in 2000. Professor David obtained a PhD on clinically applicable methods of delivering fetal gene therapy in 2005 at UCL and she established the group in 2007.
Current research projects
- The EVERREST Project - Maternal gene therapy
Fetal Growth Restriction (FGR) is an important condition which affects 8 out of 100 pregnancies and means that the baby does not grow in the womb as well as it should. At the moment there is no treatment. There is also no way to predict and prevent fetal growth restriction from occurring. Sadly some babies die in the womb and others have to be delivered prematurely. Babies who are small and premature can have serious health problems immediately after birth and as they grow; some babies may even die after birth.
The EVERREST project is aiming to develop a treatment for fetal growth restriction. The most common cause is a lack of sufficient blood flowing to the womb via the mother’s circulation. This results in a lack of nutrients and oxygen to the developing baby. Our previous research has shown that increasing blood flow to the womb using localized maternal VEGF gene therapy can improve fetal growth. The aim of the project is to carry out the first trial of this therapy in pregnant women whose babies are most severely affected by fetal growth restriction to test out its safety and efficacy. Potential benefits from the research could include reduced stillbirths and neonatal deaths, and improved neonatal and long term outcomes in pregnancies affected by severe early onset fetal growth restriction. To find more about Abigail, who had fetal growth restriction, please follow the link below.
- Project Coordinator : Professor Anna David
- Professor and Consultant in Obstetrics and Maternal Fetal Medicine
EGA UCL Institute for Women's Health, University College London,
Room 244, Huntley Street, London WC1E 6AU, United Kingdom
Email : a.david@ucl.ac.uk
Trial Email : everresttrial@ucl.ac.uk
- Fetal gene therapy
In utero Gene Therapy (IUGT) for Congenital Blood Disorders
Beta thalassaemia is a genetic blood disease that causes life-threatening anemia. Hematopoietic stem cell (HSC) transplantation successfully cures the disease but in only 30% of patients.
In humans, successes with in utero transplantation using allogeneic haematopoietic stem cells, has been limited to fetuses with severe immunologic defects where there is an effective lack of immune response to allogeneic cells.
We hypothesized that IUGT to the fetal HSC compartment (Liver) using a vector carrying the corrected beta globin gene (GLOBE, Dr Mike Antoniou Kings College London) might cure the disease before birth.
Ideally, having a diagnosis using Non Invasive Prenatal Diagnosis (NIPD) at 10 weeks of gestation will give enough time to administer IUGT and correct the disease before birth.

Team: Panicos Shangaris, Stavros P Loukogeorgakis, Sindhu Subramaniam, Christina Flouri, Simon Waddington
Funding: Wellcome Trust, Sparks, UCLH Charities, UKTS
- Fetal stem cell gene therapy
Overview: Inherited genetic disorders are important causes of morbidity and mortality. Prenatal diagnosis is now possible early in pregnancy using the maternal blood. Postnatal stem cell transplantation is limited by the immune response to the cells. Stem cells in the amniotic fluid (AF) cells may be a good cell source for prenatal therapy. Some of these AF stem cells have hematopoietic activity meaning they may be used to treat inherited blood disorders such as thalassaemia or sickle cell anaemia. We are studying how to use AF stem cells to correct inherited anaemias before birth.
Design:
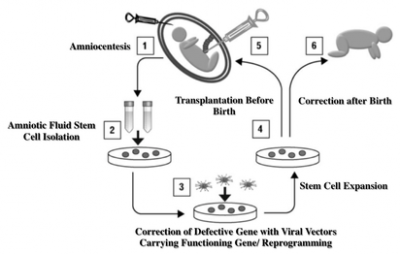
Team: Durrgah L Ramachandra, Stavros P Loukogeorgakis, Dr Panicos Shangaris, Eleni Antoniadou, Sindhu Subramaniam.
Funding: Wellcome Trust, Sparks, Rosetrees Trust
Links: Wellcome Trust, Sparks, Rosetrees Trust, UK Thalassaemia Society
- In utero stem cell transplantation
Overview: Osteogenesis imperfecta (OI) is an inherited genetic disease characterised by brittle bones. When severe, babies present in the womb with fractures that can be seen on ultrasound scan. Patients with severe OI have repeated, multiple fractures through life, pain and handicap. There is no cure and current treatment is palliative. Fetal therapy using stem cells presents an opportunity. Transplantation before birth at the onset of disease may give better engraftment with less rejection than transplantation after birth. Mesenchymal stem cells (MSC) are promising candidates with minimal cancer risk.
Our aim is to develop prenatal and postnatal transplantation of MSC as a therapy for severe types of OI.
Design: We are conducting a multicentre case control study of MSC transplantation in severe types of OI (types II/III, severe type IV). We will compare prenatal (in utero) and postnatal transplantation and evaluate safety and efficacy outcomes (fracture frequency and growth). The multicentre study is lead by Sweden, with three other clinical centres at UCL, Koln in Germany, and Leiden in The Netherlands. We are also working with Great Ormond Street Hospital to develop non-invasive prenatal testing for OI.
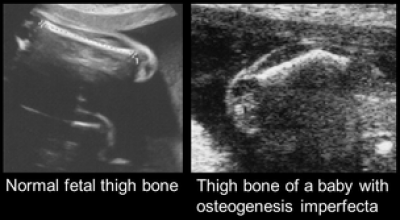
Team: Dr Anna David, Dr Pascale Guillot, Professor Mark Lowdell (UCL Royal Free Biobank), Professor Lyn Chitty (Institute of Child Health), Dr Catherine De Vile (Great Ormond Street Hospital).
Funding: “Stem cell therapy to treat severe osteogenesis imperfecta”. Swedish Krona 16,500,000 (£1,357,887) over 3 years. Funded by a “Framework grant in clinical therapy research” from Swedish Research Council. Collaboration with Dr Cecilia Götherström and Professor Magnus Westgren.
Links: BOOSTB4
- Healing the amniotic membrane
Overview: Pre-term prelabour rupture of the fetal membrane (PPROM) is a major cause of preterm birth. It accounts for 40 % of early infant death and costs the NHS £3 billion annually. Infection, blood and uterine stretch weaken the membrane. Little is known about the factors which initiate the damaging process. PPROM occurs commonly after prenatal fetoscopic and open fetal surgery for identical twin problems and fetal abnormalities. Spontaneous wound healing of the amniotic membrane (AM) does not occur after invasive fetal therapy. A visible membrane defect is seen in the majority of cases. Attempts to seal the defect with glues or decellularised collagen plugs are not clinically effective. Fetal therapy is therefore limited by the complications of prematurity.
Design: We are examining the role of the stretch-sensitive protein connexin-43 (Cx43) in fetal membrane healing and tissue strength. We are also studying the effect of cyclic tensile strain on Cx43 expression and production of inflammatory mediators. With the help of material scientists our aim is to develop a novel bioactive sealing method to heal fetal membrane defects after fetal surgery.
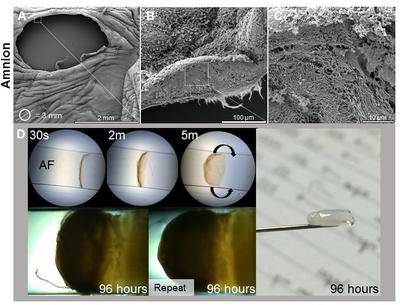
(A-C) Microstructure analysis for the human amniotic membrane following fetoscopy surgery the defect and fibrous architecture of the exposed fetal membrane. (D) Development of novel sealing strategy based on self-assembling membranes at the amniotic fluid interface.
Team: Eleni Costa (PhD student), Dr Chris Thrasivoulou (UCL), Professor David Becker (UCL), Dr. Tina Chowdhury and Dr Babatunde Okesola (Queen Mary University of London), Prof. Jan Deprest (University of Leuven, Belgium) and Prof. Che Connon (University of Newcastle).
Funding: Rosetrees Trust, Queen Mary University of London, Prenatal Therapy Charity
- Biobanking fetal stem cells for therapy
Overview: Human amniotic fluid stem cells (hAFSC) rapidly expand and differentiate into many types of cell. They have a low risk for causing tumours. They can easily be collected at prenatal diagnosis or at birth. We are establishing an experimental pathway for biobanking human AFSCs for clinical use under GMP conditions. In the future, we hope to test their therapeutic efficacy for intestinal diseases such as Necrotizing Enterocolitis (NEC) a common serious disease of preterm born neonates.
Design: We are processing and charactering stem cells in the human amniotic fluid for growth yield, population purity, cellular potency, and viability, using standard culture conditions and xeno-free (animal-cell free) culture conditions.
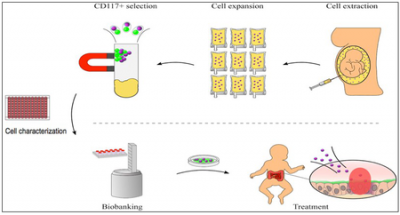
The roadmap from AFSC biobanking to NEC treatment.
Team: Eleni Antoniadou, Durrgah Ramachandra, Sindhu Subramaniam
PI: Dr Anna David, Professor Paolo De Coppi, Professor Mark Lowdell
Funding: Rosetrees Trust, Stoneygate Trust, NIHR Great Ormond Street Hospital UCL Institute of Child Health Biomedical Research Centre
- Imaging for fetal therapy
Overview: 1% of babies are born with severe defects. These are collectively responsible for over 1/3 of all paediatric hospital admissions. Early intervention, when the baby is still in the womb, in some cases improves outcome in terms of morbidity and mortality. Guided Instrument for Fetal Therapy and Surgery is a seven-year project in collaboration with KU Leuven, Great Ormond Street Hospital and University College Hospital. We aim to develop low-risk techniques for the diagnosis, treatment and therapy of a range of debilitating abnormalities of the baby during pregnancy.
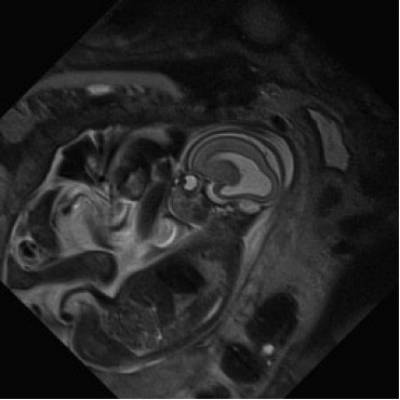
Team: Dr Rosalind Pratt, Efthymios Maneas
Funding: Guided Instrument for Fetal Therapy and Surgery is a seven-year project funded by the Wellcome Trust and Engineering and Physical Sciences Research Council.
Links: GIFT Surg, The Guardian
- Developing obstetric therapies
The development of new drugs for use in obstetrics is long overdue. Maternal and perinatal disease counts for approximately 7% of global disease, yet fewer than 5% of the number of drugs being developed to combat cardiovascular disease are designed to help new mothers and pregnant women.
Our research is developing new obstetric therapies. We have also worked with the Royal College of Obstetricians and Gynaecologists (RCOG) to suggest ways that barriers to finding new treatments could be overcome.
See the paper here - Developing New Pharmaceutical Treatments for Obstetric Conditions
Developing Maternal and Fetal Adverse Event severity grading criteria
To assess the safety of a potential new therapy, researchers record any Adverse Events (AEs) which occur during clinical trials. To get the most detailed safety data these AEs are usually graded from 1 to 5. Standard criteria exist for grading hundreds of AEs, but these are inadequate and inconsistent for trials of maternal and fetal therapies.
The EVERREST International Adverse Event Consensus Group met for the first time in May 2015 to begin the process of developing standard maternal and fetal AE severity grading criteria. This group includes fetal therapy, obstetric, neonatal, and pharmaceutical industry experts from Europe and the United States. The first set of criteria were adopted by MedDRA, the Medical Dictionary of Regulatory Activities in their updated Version 19.0 in March 2016.

- Ethics of prenatal therapies
Ethics of prenatal therapies
Overview: Ethical considerations are an important part of developing prenatal therapies and are embedded in all our research. We have investigated ethical issues in relation to prenatal gene and cell therapy for genetic and obstetric conditions with stakeholders and potential patients.
Maternal gene therapy:
We considered the ethics of placental gene therapy here.
A recent review suggests that there is no objection of ethical, legal or regulatory principles to the maternal gene therapy intervention under development in the EVERREST programme.
Fetal surgery:
We are reviewing the ethical, legal and regulatory principles relating to novel fetal surgery.
Collaborations: Professor Richard Ashcroft (Queen Mary University of London); Professor Jan Deprest (UCL); Dr Kevin Cao, Alice Booth
Funding: European Commission, Wellcome Trust, EPSRC
- Public engagement
Public engagement
Engaging with Patients
Overview: Involving patients in designing research pathways helps to achieve successful outcomes and is an important aspect of our research. The group engages with the public and patients in the following ways:
- Identifying patient priorities for research
- Discussing ethical issues relating to developing prenatal therapy
- Understanding issues of informed consent relating to trials of prenatal therapy
- Collaborating with patients to develop new ways to image and treat the fetus in the womb
Team: Preterm Birth
Anna David and Catherine James are members of the steering group for the Preterm Birth Priority Setting Partnership, James Lind Alliance. In conjunction with families with experience of preterm birth and organisations representing them we identified and prioritised the top 15 research questions of which the top is “Which interventions are most effective to predict or prevent preterm birth?”
Team: Prenatal Imaging and Surgery
The Patient Public Advisory Group for GIFT-Surg was set up as part of a 7 year programme grant funded by a Wellcome Trust and EPSRC Innovative Engineering for Health Award. The group has parents and representatives from a number of UK charities (SHINE; CDH-UK; BLISS; TAMBA; ARC) who are providing continuous input to the project during the development of innovations.
Team: Maternal Gene Therapy for Fetal Growth Restriction
As part of the 6 year EVERREST programme, we consulted with patients who had experienced a pregnancy with severe early onset fetal growth restriction to understand their views about developing a treatment. The overall conclusion drawn from these interviews was that they had a generally favourable view of the ethical and social acceptability of a maternal gene therapy to treat this condition.
Team: Prenatal Treatment of Congenital Disease
We are working with groups such as the UK Thalassaemia Society and The Sickle Cell Society to develop new treatments for these common blood disorders and to inform them of our work.
Links:
GIFT-Surg project: www.gift-surg.ac.uk
ARC – Antenatal Results and Choices: www.arc-uk.org
Bliss – for babies born to too soon, too small, too sick: www.bliss.org.uk
CDH UK – the Congenital Diaphragmatic Hernia Support Charity: www.cdhuk.org.uk
Shine – Spina bifida and Hydrocephalus information, networking and equality: www.shinecharity.org.uk
TAMBA – Twin and multiple birth association: www.tamba.org.uk
UK Thalassaemia Society: www.ukts.org
Sickle Cell Society: www.sicklecellsociety.org
- Prenatal therapy charity
Prenatal therapy charity
The Prenatal Therapy Fund was set up in December 2013 to support research in the group.

To date the funds have supported:
- A collaborative lab visit to Belgium to develop new techniques to plug the amniotic membrane after fetoscopic surgery.
- Microscopic analysis of amniotic membrane after fetal therapy.
- Analysis of haemoglobin levels after fetal gene therapy for thalassaemia.
- A microscope to study bacteria in the urine of women at high risk of spontaneous preterm birth.
The fund has a webpage on Just Giving. We are also supported by “Little heartbeats raises for UCLH heal membranes project”.
All funds raised go directly to support research into developing prenatal therapies.

You can donate online at the UCLH Prenatal Therapy Justgiving page
- News
News
Our work has been featured in The Times, New Scientist , BBC Future Health and on YouTube.
Featured news:
UCL/ParisDescartes University Collaborative
The objective of the project is to initiate a UCL – Paris Descartes Collaborative program in Women’s Health. The UCL- Paris Descartes Collaborative will be launched in May 2017 with a symposium involving the two university departments and their affiliated hospital systems. This faculty-led initiative originating in women’s health medicine could expand to other biomedical fields and disciplines in due course. UCL and Paris Descartes present both a great environment for care, research and teaching, with many similarities but also many differences. Students, carers and scientists could all highly benefit from this collaboration. The objective of the Collaborative is therefore to create deep interactions and broader relationship between the two institutions and also to take better advantage of complementary resources. This will be built on seminars, workshops, visits for lectures, joint projects and on exchanges of medical students. Professorships positions could also be created. This project is also supported by the French Embassy in London.
Thanks to the Co-Op for raising £6,382 for our research to find a way to heal the amniotic membranes

- Second International Meeting on In Utero Transplantation and Fetal Gene Therapy, London, UK 9th-10th October 2015. Learn about advanced fetal therapy, stem cells, regenerative medicine and prenatal gene therapy. The theme is "Translating fetal stem cell and gene therapies into the clinic". http://www.ucl.ac.uk/ich/education/events/the-second-international-conference-on-in-utero-transplantation-and-fetal-gene-therapy-2015
- UCL Lunch Hour Lecture entitled “How can we improve growth of small babies before birth?” as part of the Institute for Women’s Health celebrations of International Women’s Day in 2015. https://www.youtube.com/watch?v=LNDJnI6MRL8
- In the first TEDx UCLwomen event 6th December 2013 http://tedxuclwomen.com/ Anna spoke on “The third option: treating disease in babies before birth”. http://tedxuclwomen.com/portfolio/anna-david/; https://www.youtube.com/watch?v=CzsN7D4Y-5I
- Research into amniotic fluid stem cells in collaboration with Professor Paolo De Coppi at UCL Institute of Child Health was made specially to mark International Stem Cell Awareness Day, 5 October 2011. The day aims to promote greater understanding about stem cell research, the range of potential applications for disease and injury, and to raise awareness about why funding stem cell research is so vital. http://www.youtube.com/watch?v=qD_V1hqR6SI
@PrenatalTherapy: Obstetrician and specialist in maternal fetal medicine, with an interest in developing prenatal therapies for pregnancy complications and congenital disease
@FP7EVERREST: Does vascular endothelial growth factor gene therapy safely improve outcome in severe early-onset fetal growth restriction?
 Close
Close


