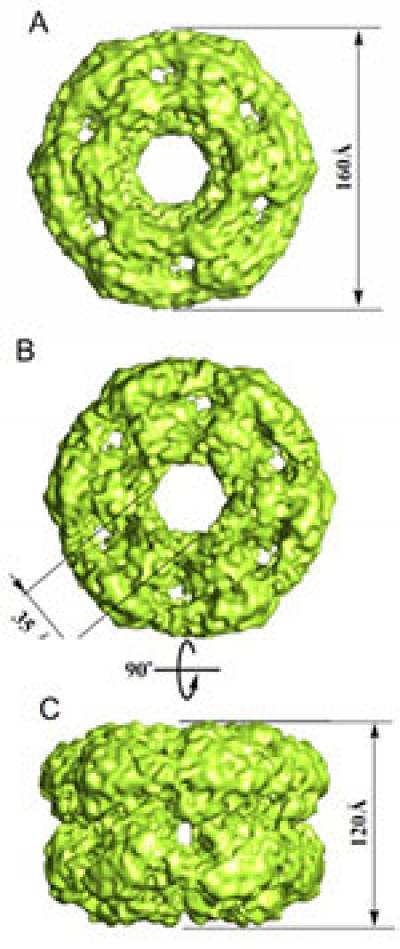
The DNA replication factor minichromosome maintenance 10 (MCM10) is a conserved, abundant nuclear protein crucial for origin firing. During the transition from pre-replicative complexes to pre-initiation complexes, MCM10 recruitment to replication origins is required to provide a physical link between the MCM2-7 complex DNA helicase and DNA polymerases. We have recently solved the molecular structure of human MCM10 as determined by electron microscopy and single-particle analysis. The MCM10 molecule is a ring-shaped hexamer with large central and smaller lateral channels and a system of inner chambers. The structure, together with biochemical data, suggests that this important protein uses its architecture to provide a docking module for assembly of the molecular machinery required for eukaryotic DNA replication.
The MCM10 molecule shows remarkable structural similarity to DNA helicases, but has no conserved helicase motifs within its sequence and no detectable DNA helicase activity in vitro. It is possible that as a renegade helicase, MCM10 has lost its activity for a structural supportive role as a 'docking' module aimed to facilitate protein-protein interactions between DNA replication proteins. The ring shape is ideal in providing a structural basis for MCM10 interaction with essential replication factors simultaneously, perhaps linking the Mcm2-7 helicase ring on one side and DNA Pol-α primase on the other. Taken together, the ring-shaped architecture and ability of MCM10 to bind to DNA in a sliding clamp manner suggest a possible molecular mechanism by which MCM10 facilitates protein-protein interactions during the pre-RC to pre-IC transition and later during the elongation stage. The putative arrangement of DNA within the MCM10 ring would provide for processivity and free movement on DNA, and thus could explain the reported MCM10 requirement for fork progression by maintaining contact between the replicative helicase and polymerases
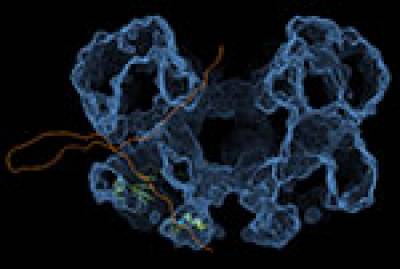
Figure 3. 3D EM map of MCM10 shows double-layered ring assembly with a sixfold symmetry. Left panel - model for ssDNA threading through the MCM10 hexamer. ssDNA (orange) enters via central channel contacting the Zn-finger of the cenral OB-fold domain (3EBE; in green) binds the hydrophobic patch of the OB- domain, exits side channel near the a-polymerase binding side, loops and reenters central channel, whereas interactingwith C-terminal Zn-fingers
Okorokov AL, Waugh A, Hodgkinson J, et al. (2007) Hexameric ring structure of human MCM10 DNA replication factor. EMBO Reports 8:925-930. free access
 Close
Close