Surgical Interventional Group
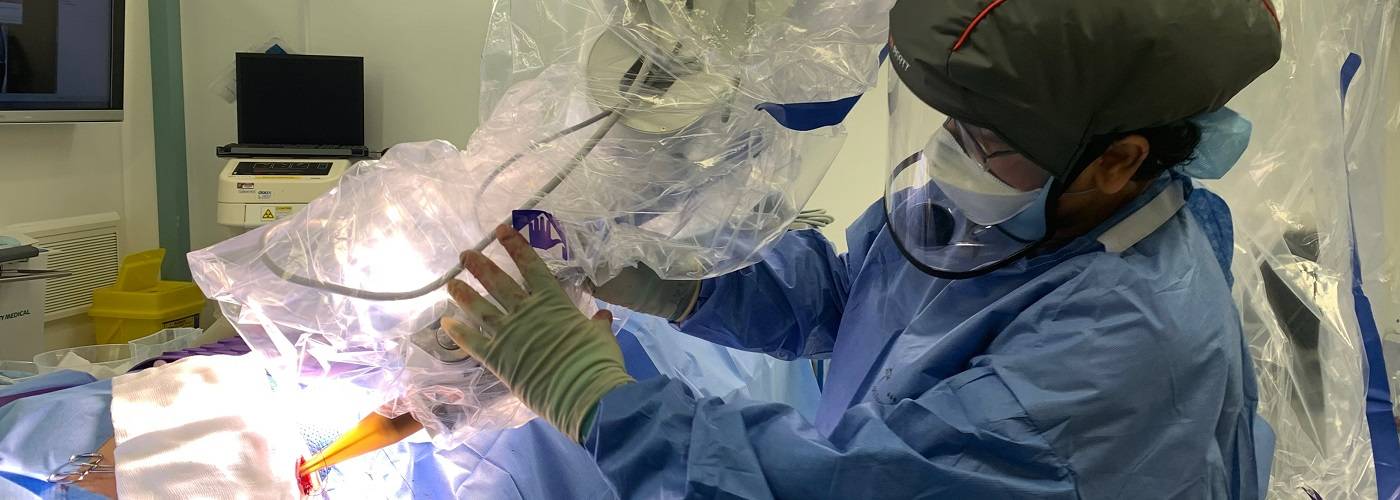
REF 2021 Highlight: Single-dose radiotherapy transforms breast cancer patient care
Our clinicians have transformed breast cancer care by developing a single-dose radiotherapy treatment to be delivered in the same operation as tumour surgery.

PIT-STOP Trial announced
The PIT-STOP trial from UCL Eastman investigates the effect of combining two medications to reduce soft tissue fibrosis of the mouth and throat in head and neck cancer survivors.
The Surgical & Interventional Group is part of UCL's accredited Comprehensive Clinical Trials Unit. Located in Bloomsbury, London, we specialise in providing support to run studies led by surgical and interventional researchers.
Working with clinicians, statisticians, qualitative researchers, health economists, and patient and public representatives (PPI), we provide expertise and support for the design and conduct of high-quality clinical studies aimed at guiding clinical practice.
Publications
- Kasivisvanathan, V., Emberton, M., Moore, C.M. et al. for the PRECISION Study Group Collaborators (2018). MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. The New England Journal of Medicine.
- ATAC Trialists' Group, Forbes JF, Cuzick J, Buzdar A, et al. (2008). Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. The Lancet Oncology, Vol. 9, Issue 1, pp. 45-53.
- Vaidya J.S., Wenz F., Bulsara M., Tobias J.S. et al. on behalf of the TARGIT trialist's group (2014). Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomised trial. The Lancet, Vol. 383, Issue 9917, pp. 603-613.
- Current Trials Working Party of the Cancer Research Campaign Breast Cancer Trials Group (1996). Preliminary results from the cancer research campaign trial evaluating tamoxifen duration in women aged fifty years or older with breast cancer. JNCI: Journal of the National Cancer Institute, Vol. 88, Issue 24, pp. 1834–1839.
- 2021
- Fabes J, Ambler G, Shah B, Williams NR, Martin D, Davidson BR, Spiro M. Protocol for a prospective double-blind, randomised, placebo-controlled feasibility trial of octreotide infusion during liver transplantation. BMJ Open. 2021 Dec 2;11(12):e055864.
- Treasure T, Farewell V, Macbeth F, Batchelor T, Brew-Graves C, et al. The Pulmonary Metastasectomy in Colorectal Cancer (PulMiCC) burden of care study: Analysis of local treatments for lung metastases and systemic chemotherapy in 220 patients in the PulMiCC cohort. Colorectal Dis. 2021 Nov;23(11):2911-2922.
- Vaidya JS, Bulsara M, Baum M, Wenz F, Massarut S, Pigorsch S, et al. New clinical and biological insights from the international TARGIT-A randomised trial of targeted intraoperative radiotherapy during lumpectomy for breast cancer. Br J Cancer. 2021 Aug;125(3):380-389.
- Dinneen E, Haider A, Grierson J, Freeman A, Williams NR, Brew-Graves C, et al. NeuroSAFE frozen section during robot-assisted radical prostatectomy: peri-operative and histopathological outcomes from the NeuroSAFE PROOF feasibility randomized controlled trial. BJU Int. 2021 Jun;127(6):676-686.
- Orczyk C, Barratt D, Brew-Graves C, Peng Hu Y, Williams NR, Emberton M, et al. Prostate Radiofrequency Focal Ablation (ProRAFT) Trial: A Prospective Development Study Evaluating a Bipolar Radiofrequency Device to Treat Prostate Cancer. J Urol. 2021 Apr;205(4):1090-1099.
- 2020
- Brew-Graves C, Farewell V, Monson K, Milošević M, Williams NR, et al. Pulmonary metastasectomy in colorectal cancer: health utility scores by EQ-5D-3L in a randomized controlled trial show no benefit from lung metastasectomy. Colorectal Dis. 2021 Jan;23(1):200-205.
- Milosevic M, Edwards J, Tsang D, Dunning J, Shackcloth M, Batchelor T, et al. Pulmonary Metastasectomy in Colorectal Cancer: updated analysis of 93 randomized patients - control survival is much better than previously assumed. Colorectal Dis. 2020 Oct;22(10):1314-1324.
- Vaidya JS, Bulsara M, Baum M, Wenz F, Massarut S, Pigorsch S, et al. Long term survival and local control outcomes from single dose targeted intraoperative radiotherapy during lumpectomy (TARGIT-IORT) for early breast cancer: TARGIT-A randomised clinical trial. BMJ. 2020 Aug 19;370:m2836.
- Vaidya JS, Bulsara M, Saunders C, Flyger H, Tobias JS, Corica T, et al. Effect of Delayed Targeted Intraoperative Radiotherapy vs Whole-Breast Radiotherapy on Local Recurrence and Survival: Long-term Results From the TARGIT-A Randomized Clinical Trial in Early Breast Cancer. JAMA Oncol. 2020 Jul 1;6(7):e200249.
- Tan WS, Teo CH, Chan D, Ang KM, Heinrich M, Feber A, Sarpong R, Williams N, Brew-Graves C, Ng CJ, and Kelly J. Exploring patients' experience and perception of being diagnosed with bladder cancer: a mixed-methods approach. BJU Int. 2020 May;125(5):669-678.
- Banerjee SM, El-Sheikh S, Malhotra A, Mosse CA, Parker S, Williams NR, MacRobert AJ, Hamoudi R, Bown SG, and Keshtgar MR. Photodynamic Therapy in Primary Breast Cancer. J Clin Med. 2020 Feb 10;9(2).
- 2019
- D.S. Martin, C. Brew-Graves, N. McCartan, G. Jell, et al. (2019). Protocol for a feasibility randomised controlled trial of targeted oxygen therapy in mechanically ventilated critically ill patients. BMJ Open.
- 2018
- V. Kasivisvanathan, A.S. Rannikko, M. Borghi, V. ... C. Brew-Graves, J. Deeks, Y. Takwoingi, M. Emberton, and C.M. Moore, for the PRECISION Study Group Collaborators. (2018). MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis' The New England Journal of Medicine.
- Orczyk C, Brew-graves C, WIlliams N, Emberton M, et al. (2018). Prostate Radiogrequncy Ablation Focal Treatment (PRORAFT): Results of a prospective development study for localised prostate cancer. Presented at: American-Urological-Association (AUA) Annual Meeting.
- Grey A, Scott R, Charman S, van der Meulen J, Frinking P, Acher P, Brew-Graves C, et al. (2018). The CADMUS trial ‐ Multi‐parametric ultrasound targeted biopsies compared to multi‐parametric MRI targeted biopsies in the diagnosis of clinically significant prostate cancer. Contemp Clin Trials.
- 2017
- Williams, N. R., Kurylcio, A., Jankiewicz, M., Romanek, J., Polkowski, W., Michalopoulos, N. V., & Keshtgar, M. R. (2017). Aesthetic outcome after breast conserving surgery and either intraoperative radiotherapy or whole breast external beam radiotherapy for early breast cancer: Objective assessment of patients in a randomized controlled trial in Lublin, Poland. European Journal of Gynaecological Oncology, 38 (6), 867-870.
- Reis, S., Gazinska, P., Hipwell, J., Mertzanidou, T., Naidoo, K., Williams, N., Pinder, S., Hawkes, D.J. (2017). Automated Classification of Breast Cancer Stroma Maturity from Histological Images. IEEE Trans Biomed Eng.
- Treasure, T., Williams, N.R. (2017). Best available evidence related to clinical benefit of surgical resection in multimodality treatment of metastatic colorectal cancer indicates that a randomised controlled trial is warranted. European journal of cancer, 75, 310-312.
- Tan, W.S., Feber, A., Dong, L., Sarpong, R., Rezaee, S., Rodney, S., Khetrapal, P., de Winter, P., Ocampo, F., Jalil, R., Williams, N.R., Brew-Graves, C., Kelly, J. D. (2017). DETECT I & DETECT II: a study protocol for a prospective multicentre observational study to validate the UroMark assay for the detection of bladder cancer from urinary cells. BMC CANCER, 17, ARTN 767.
- Vaidya, A., Vaidya, P., Both, B., Brew-Graves, C., Bulsara, M. & Vaidya, J.S. (2017). Health economics of targeted intraoperative radiotherapy (TARGIT-IORT) for early breast cancer: a cost- effectiveness analysis in the United Kingdom. BMJ Open.
- Orczyk C, Brew-Graves C, Williams N, Potyka I, Ramachandran N, Freeman A, Emberton M, Ahmed HU. (2017). Prostate radiofrequency ablation focal treatment (PRORAFT): interim results of a prospective development study. Presented at: American Urological Association (AUA) 2017 Annual Meeting.
- 2016
- Vaidya, J.S., Wenz F., Bulsara M., Tobias J.S., Joseph D.J., Saunders C., Brew-Graves C., Potyka I., Morris S., Vaidya H.J., Williams N.R., Baum M.(2016). An international randomised controlled trial to compare TARGeted Intraoperative radioTherapy (TARGIT) with conventional postoperative radiotherapy after breast-conserving surgery for women with early-stage breast cancer (the TARGIT-A trial). Health Technology Assessment.
- Coombs, N.J., Coombs, J.M., Vaidya, U.J., Singer, J., Bulsara, M., Tobias, J., Wenz, F., Joseph, D. J., Brown, D. A., Rainsbury R., Davidson, T., Adamson D.J.A., Massarut S., Morgan, D., Potyka, I., Corica, T., Falzon, M., Williams N.R., Baum, M., Vaidya, J.(2016). Environmental and social benefits of the targeted intraoperative radiotherapy for breast cancer: data from UK TARGIT-A trial centres and two UK NHS hospitals offering TARGIT IORT. BMJ OPEN, 6 (5).
- Keshtgar, M., Williams, N.R. (2016). Intraoperative radiation therapy deserves to be made more readily available to patients. Chinese Journal of Cancer Research, 28 (4), 461-462.
- Mokhles, S., Macbeth, F., Farewell, V., Fiorentino, F., Williams, N.R., Younes, R.N., Takkenberg, J.J., Treasure, T. (2016). Meta-analysis of colorectal cancer follow-up after potentially curative resection. The British Journal of Surgery.
- Vavourakis, V., Eiben, B., Hipwell, J.H., Williams, N.R., Keshtgar, M., Hawkes, D.J. (2016). Multiscale Mechano-Biological Finite Element Modelling of Oncoplastic Breast Surgery-Numerical Study towards Surgical Planning and Cosmetic Outcome Prediction. PLoS ONE, 11 (7).
- Vaidya, J.S., Bulsara, M., Wenz, F., Coombs, N., Singer, J., Ebbs, S., Massarut, S., Saunders, C., Douek, M., Williams, N.R., Joseph, D., Tobias, J.S., Baum, M.(2016). Reduced Mortality With Partial Breast Irradiation for Early Breast Cancer. International Journal of Radiation Oncology Biology Physics, 96 (3), 707-708.
- Ghosh, D., Michalopoulos, N.V., Davidson, T., Wickham, F., Williams, N.R., Keshtgar, M.R. (2016). Sentinel node detection in early breast cancer with intraoperative portable gamma camera: UK experience. Breast, 32 53-59.
- Eiben, B., Vavourakis, V., Hipwell, J.H., Kabus, S., Buelow, T., Lorenz, C., Mertzanidou, T., Reis, S., Williams, N.R., Keshtgar, M., Hawkes, D.J. (2016). Symmetric Biomechanically Guided Prone-to-Supine Breast Image Registration. Annals of Biomedical Engineering, 44 (1), 154-173.
- Banerjee, S.M., Williams, N.R., Davidson, T.I., El Sheikh, S., Tran-Dang, M., Davison, S., Ghosh, D., Keshtgar, M.R.S. (2016). The use of onestep nucleic acid amplification (OSNA) and tumour related factors in the treatment of axillary breast cancer: A predictive model. European Journal of Surgical Oncology, 42 (5), 641-649.
- Winters, Z.E., Horsnell, J., Schmid, P., Jones, J.L., Shaaban, A., Maxwell, A.J., Williams, N.R., Esserman, L., Jatoi, I., Brunt, A.M. (2016). Time for a randomised clinical trial evaluating breast conserving surgery compared to mastectomy in ipsilateral mutlifocal breast cancer (MFBC)? Breast, 26 149-150.
- 2015
- Cardillo, G., Mokhles, S., Williams, N., Macbeth, F., Russell, C., Treasure, T. (2015). Comment on: 'KRAS and BRAF mutations are prognostic biomarkers in patients undergoing lung metastasectomy of colorectal cancer.' Variation in survival associated with proto-oncongenes is not evidence for effectiveness of lung metastasectomy. British Journal of Cancer, 113 (11), 1636.
- Vaidya, J. S., Bulsara, M., Wenz, F., Joseph, D., Saunders, C., Massarut, S., Flyger, H., Eiermann, W., Alvarado, M., Esserman, L., Falzon, M., Brew-Graves, C., Potyka, I., Tobias, J., Baum, M. (2015). In Regard to Hepel and Wazer. International Journal of Radiation Oncology Biology Physics, 92(5), 953-954.
- Vaidya, J. S., Bulsara, M., Wenz, F., Joseph, D., Saunders, C., Massarut, S., Flyger, H., Eiermann, W., Alvarado, M., Esserman, L., Falzon, M., Brew-Graves, C., Potyka, I., Tobias, J., Baum, M. (2015). Pride, Prejudice, or Science: Attitudes Towards the Results of the TARGIT-A Trial of Targeted Intraoperative Radiation Therapy for Breast Cancer. International Journal of Radiation Oncology Biology Physics, 92(3), 491-497.
Hear from experts and patients
Prof. Jayant S. Vaidya and a trial patient, the writer Marcelle Bernstein, speak about TARGIT-IORT at a breast cancer event held at the Royal Society.
Contact
Address
Third floor
Charles Bell House
43-45 Foley Street
London
W1W 7JN
Email / Telephone
+44 (0)20 7679 9280
(Internal: 09280)
Fax: +44 (0)20 7679 9290
 Close
Close