Please click on the links below to take you directly to the specific area:
- Investigating the mechanisms underlying bariatric surgery
- Gaining insights into how the gut-brain axis regulates feeding behaviour
- Gut hormones and exercise
- Insights from genetics
- Collaborative projects
Investigating the mechanisms underlying bariatric surgery
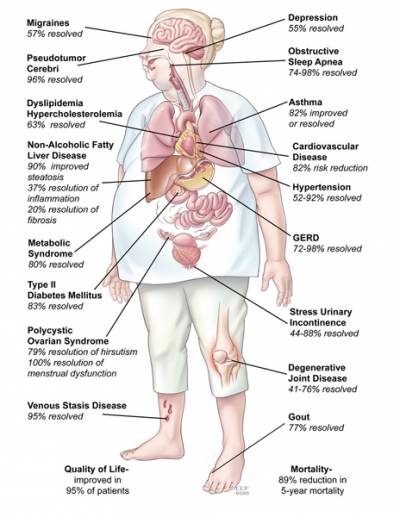
Bariatric surgery is the most effective treatment option for obesity resulting in substantial and durable weight loss, with reduction in mortality and obesity-related co-morbidities.
Obesity is the primary risk factor for type 2 diabetes mellitus (T2DM) which is characterised by insufficient insulin secretion and insulin resistance. Proximal gastric bypass surgery (PGBP) and sleeve gastrectomy (SG) reduce excess bodyweight by up to 80%.
Additionally, the majority of individuals with T2DM that undergo PGBP show immediate improved glycaemic control following surgery, before significant weight loss.
Whilst many therapeutic regimens aim to modulate glucose concentrations in order to prevent end-organ damage, bariatric surgery seems to be the only treatment modality with the potential to induce complete resolution of T2DM, however the mechanisms underlying this process remain elusive.
Understanding how these surgical procedures induce sustained weight loss and resolution of T2DM holds the key to development of more directed, less invasive therapies and is one of our key research focuses.
There is increasing evidence that the reduced appetite and improved glucose homeostasis observed in the early post-operative period after certain types of bariatric surgery is due to alterations in circulating gut hormones.
In order to gain further understanding of these processes we undertake studies on patients undergoing bariatric surgery at UCLH. In addition, we have established a mouse model of bariatric surgery to enable us to gain further mechanistic insights.
Key publications:
- Chandarana K, Gelegen C, Irvine EE, Choudhury AI, Amouyal C, Andreelli F, Withers DJ, Batterham RL: Peripheral activation of the Y2-receptor promotes secretion of GLP-1 and improves glucose tolerance. Molecular Metabolism, 2: 142-152, 2013
- Yousseif A, Emmanuel J, Karra E, Millet Q, Elkalaawy M, Jenkinson A, Hashemi M, Adamo M, Finer N, Fiennes A, Withers DJ, Batterham RL: Differential Effects of Laparoscopic Sleeve Gastrectomy and Laparoscopic Gastric Bypass on Appetite, Circulating Acyl-ghrelin, Peptide YY3-36 and Active GLP-1 Levels in Non-diabetic Humans. Obesity Surgery:1-12, 2013 DOI 10.1007/s11695-013-1066-0
- Manning SB, Pucci A, Batterham RL, Finer N: Latent autoimmune diabetes in adults presenting as diabetes "recurrence" after bariatric surgery: a case report. Diabetes Care 36:e120, 2013
- Scott WR, Gelegen C, Chandarana K, Karra E, Yousseif A, Amouyal C, Choudhury AI, Andreelli F, Withers DJ, Batterham RL: Differential pre-mRNA splicing regulates Nnat isoforms in the hypothalamus after gastric bypass surgery in mice. PLoS One 8:e59407, 2013
- Gelegen C, Chandarana K, Choudhury AI, Al-Qassab H, Evans IM, Irvine EE, Hyde CB, Claret M, Andreelli F, Sloan SE, Leiter AB, Withers DJ, Batterham RL: Regulation of hindbrain Pyy expression by acute food deprivation, prolonged caloric restriction, and weight loss surgery in mice. Am J Physiol Endocrinol Metab 303:E659-668, 2012
- Chandarana K, Batterham RL: Shedding pounds after going under the knife: metabolic insights from cutting the gut. Nat Med 18:668-669, 2012
- Chandarana K, Gelegen C, Karra E, Choudhury AI, Drew ME, Fauveau V, Viollet B, Andreelli F, Withers DJ, Batterham RL: Diet and gastrointestinal bypass-induced weight loss: the roles of ghrelin and peptide YY. Diabetes 60:810-818, 2011
- Scott WR, Batterham RL: Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: understanding weight loss and improvements in type 2 diabetes after bariatric surgery. Am J Physiol Regul Integr Comp Physiol 301:R15-27, 2011
- Karra E, Yousseif A, Batterham RL: Mechanisms facilitating weight loss and resolution of type 2 diabetes following bariatric surgery. Trends Endocrinol Metab 21:337-344, 2010.
- Rahman S, Scobie AI, Elkalaawy M, Bidlake LE, Fiennes AG, Batterham RL: Can glucose make you faint? Lancet 372:1358, 2008
Gaining insights into how the gut-brain axis regulates feeding behaviour
Complex interrelated neuronal circuits have developed in the mammalian brain to regulate many aspects of feeding behaviour. An increased understanding of how peripheral energy signals act upon these circuits to regulate food intake is essential for effective treatment of the current obesity crisis. We have shown that the gut hormone peptide YY (PYY) regulates feeding behaviour in rodents and humans and identified that the neuropeptide Y2 receptor is crucial for the anorectic effects of PYY. By generating mice-lacking PYY we have shown that this hormone plays a crucial role in the regulation of body weight. Moreover, we have shown that infusion of PYY reduces food intake in obese human subjects. Using fMRI in healthy male volunteers we have shown that PYY modulates neuronal activity within both homeostatic (hypothalamic and brainstem) and hedonic (orbitofrontal cortex) brain regions. More recently, again using fMRI we have shown that the neural response to visual food images within key brain appetite and reward brain regions in markedly altered in normal weight subjects carrying two copies of the obesity-risk FTO gene variant.
Key publications
- Karra E, O'Daly OG, Choudhury AI, Yousseif A, Millership S, Neary MT, Scott WR, Chandarana K, Manning S, Hess ME, Iwakura H, Akamizu T, Millet Q, Gelegen C, Drew ME, Rahman S, Emmanuel JJ, Williams SC, Ruther UU, Bruning JC, Withers DJ, Zelaya FO, Batterham RL: A link between FTO, ghrelin, and impaired brain food-cue responsivity. J Clin Invest 123:3539-3551, 2013
- Neary MT, Batterham RL: Gaining new insights into food reward with functional neuroimaging. Forum Nutr 63:152-163, 2010
- Batterham RL, ffytche DH, Rosenthal JM, Zelaya FO, Barker GJ, Withers DJ, Williams SC: PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature 450:106-109, 2007
Gut hormones and exercise
In collaboration with Dr David Stensel's group at Loughborough University, we have undertaken a series of studies that have investigated the effects of different types of exercise (aerobic or resistance) and also exercise intensity on appetite and gut hormones. We have found that aerobic exercise causes the greatest suppression of hunger and leads to a more favorable profile of appetite regulating hormones (lower ghrelin and higher PYY). In addition, we have shown that high intensity intermittent exercise produces greater appetite suppression with concomitant higher circulating PYY3-36 concentrations than energy-matched continuous exercise. We are now investigating the mechanisms underlying these gut hormone changes.
Key references
- Deighton K, Karra E, Batterham RL, Stensel DJ: Appetite, energy intake, and PYY3-36 responses to energy-matched continuous exercise and submaximal high-intensity exercise. Appl Physiol Nutr Metab 38:947-952, 2013
- Wasse LK, Sunderland C, King JA, Batterham RL, Stensel DJ: Influence of rest and exercise at a simulated altitude of 4,000 m on appetite, energy intake, and plasma concentrations of acylated ghrelin and peptide YY. J Appl Physiol 112:552-559, 2012
- King JA, Wasse LK, Ewens J, Crystallis K, Emmanuel J, Batterham RL, Stensel DJ: Differential acylated ghrelin, peptide YY3-36, appetite, and food intake responses to equivalent energy deficits created by exercise and food restriction. J Clin Endocrinol Metab 96:1114-1121, 2011
- Broom DR, Batterham RL, King JA, Stensel DJ: Influence of resistance and aerobic exercise on hunger, circulating levels of acylated ghrelin, and peptide YY in healthy males. Am J Physiol Regul Integr Comp Physiol 296:R29-35, 2009
Insights from genetics
Genome wide association studies have identified several obesity SNPs. However, the mechanisms by which these SNPs mediate their effects on bodyweight are unclear. We are undertaking detailed phenotype/genotype studies in normal weight subjects and obese patients to gain insights into the biology of obesity risk.
Key references
- Mägi R, Manning S, Yousseif A, Pucci A, Santini F, Karra E, Querci G, Pelosini C, McCarthy MI, Lindgren CM, Batterham RL: Contribution of 32 GWAS-Identified Common Variants to Severe Obesity in European Adults Referred for Bariatric Surgery. PLoS One 8:e70735, 2013
- Karra E, O'Daly OG, Choudhury AI, Yousseif A, Millership S, Neary MT, Scott WR, Chandarana K, Manning S, Hess ME, Iwakura H, Akamizu T, Millet Q, Gelegen C, Drew ME, Rahman S, Emmanuel JJ, Williams SC, Ruther UU, Bruning JC, Withers DJ, Zelaya FO, Batterham RL: A link between FTO, ghrelin, and impaired brain food-cue responsivity. J Clin Invest 123:3539-3551, 2013
Collaborative projects: UCL/UCLH
Dr M. Banks (Gastroenterology), Professor J. Deanfield (Institute of Cardiovascular Science), Professor R. Dolan (Wellcome Trust Center for Neuroimaging, UCL), Professor N. Donos (Eastman Dental), Mr P. Hardiman (Women's Health), Professor H. Montgomery (Medicine), Dr A Jones (Institute of Cardiovascular Science), Professor R. Viner (Adolescent Health), Professor J. Wardle (Epidemiology and Public Health).
Collaborative projects: External
PI on MRC International Mouse Phenotyping Consortium (Cambridge University, Oxford University and Imperial College), Dr D. Stensel (Loughborough University, UK), Professor F. Gribble (Cambridge University. UK), Professor S.C. Williams (King's College University London, UK), Professor D.J. Withers (Imperial College London, UK), Professor M. McCarthy (Oxford University UK), Dr F. Andreelli (Cochin Institute, Paris, France), Professor F. Santini (Pisa University), Dr R. Magi (Estonia University, Estonia), Professor J. Bruening (University of Cologne, Germany), Dr A. Leiter (University of Massachusetts, USA), Dr C. Lindgren (Broad Institute of MIT and Harvard, Boston USA), and Dr H. Iwakura (Kyoto University Hospital, Japan).
Research Funding
Rosetrees Trust, Medical Research Council, Wellcome Trust and UCLH/UCL Comprehensive Biomedical Research Centre.
 Close
Close


