Neuroscience, Physiology and Pharmacology
Welcome
From founding the first department of Physiology in England in 1828 to its groundbreaking work in Pharmacology to its current position as part of the largest research community of Neuroscientists in Europe, this research department has been instrumental in the world's understanding of the nervous system and the interactions within it.
From James Black to Archibald Hill to Bernard Katz, UCL has been home to Nobel Prize winners and game changers in the discipline of physiology.
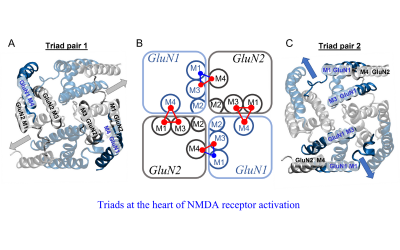
Today NPP continues this fine tradition with some of the most prestigious academics in the field. From Maria Fitzgerald and her groundbreaking work on pain to David Attwell's important work on the control of blood flow to Annette Dolphin's work on how neurons talk to each other and many more, UCL's many NPP research labs are generating exciting new findings daily.
We are proud of our focus on research-based teaching and offering the top neuroscientists of tomorrow the opportunity to hear from the current leaders in the field on their areas of expertise as well as ensuring that practical experience in labs remains a fundamental element of the teaching process.
Stephanie Schorge - Head of Department
Our people and our history
Research
Study
 Close
Close