Seeing elements transform at the atomic scale for the first time
15 June 2015
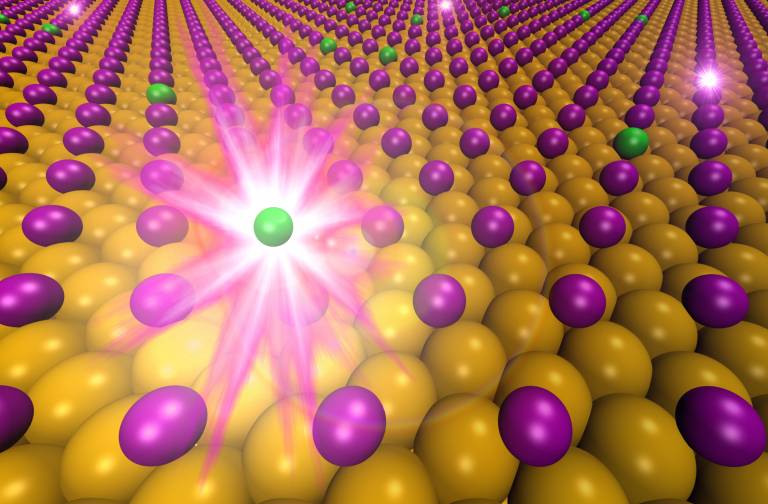
Atoms of one chemical element morphing into another have been observed for the first time.
The research, published today in the journal Nature Materials, has unexpectedly revealed a new, safer way to potentially treat cancer with radiation.
The work was done as a collaboration between the researchers at UCL, Tufts University's School of Arts and Sciences, and PerkinElmer Inc, and shows how a radioactive isotope routinely used in cancer therapies - iodine-125 - was visualised at the atomic level. As each atom of iodine-125 decayed, it lost a proton and became a non-radioactive isotope of the element - tellurium-125.
The transformation of one element to another occurred when a single droplet of water was infused with iodine-125 and deposited on a thin layer of gold. When the water evaporated, the iodine atoms bonded with the gold. A tiny sample - smaller than a five pence piece - was then studied using a combination of scanning tunnelling microscopy and computer simulations.
Iodine-125 atoms have a half-life of 59 days. This means that at any time, any of the iodine-125 atoms will decay, giving off vast amounts of energy to become isotopes of tellurium, with half of the atoms decaying every 59 days. It was impossible to predict when any one of the trillions of atoms in the sample would change into tellurium so the researchers at Tufts watched the atoms round the clock for several weeks. Eventually they managed to take scanning tunnelling microscope images that showed small atom-sized spots all over the surface.
UCL researchers then used state of the art quantum mechanical approaches to simulate images of what an iodine layer dotted with tellurium should look like and use this to explain the very high stability of the radioactive film.
Professor Angelos Michaelides (London Centre for Nanotechnology at UCL and the Thomas Young Centre), said: "The experiments performed in Charlie Sykes' lab in Tufts are absolutely outstanding and when we were shown the preliminary results, we wanted to see how we could help test their claims using quantum mechanics methods. From our simulations, we were able to show that the radioactive decay product was indeed tellurium, proving they had succeeded in seeing one atom transform into another."
PhD student Philipp Pedevilla (UCL Chemistry), said: "The experimental methods used by our collaborators directly exploit the quantum nature of electrons. It was therefore crucial to describe these electrons with a quantum mechanical framework, which we did using state-of-the-art theoretical approaches combined with the power of supercomputers. Pushing the boundaries of what we know by merging extraordinary experiments with modern physical theories was very exciting. We are now collaborating on explaining what happens to the tellurium atoms after the decay."
Tufts then studied the emission of low-energy electrons in more depth. These electrons are very effective in treating cancers because they damage the cancer cells' DNA and do not travel very far (only 1-2 nm), so can be targeted at tumours and do not affect nearby healthy tissues. They found that iodine-125 bound to gold emitted six times as many low-energy electrons as iodine-125 on its own, making it a more effective way of delivering a localised radiation dose to cancers.
Lead researcher, Professor Charles Sykes (Tufts University), said: "The gold acts like a reflector and an amplifier. Every surface scientist knows that if you shine any kind of radiation on a metal, you get this big flux of low-energy electrons coming out."
Professor Sykes' team is now planning to study iodine-125 coated gold nanoparticles with antibodies attached that target malignant tumours. The aim is to deliver the nanoparticles to cancer patients using a single injection, whereby the nanoparticles will attach to the tumour using the antibodies and emit low-energy electrons, destroying the tumour's DNA. The gold particles would then be flushed out of the body, unlike free iodine-125 which can accumulate in the thyroid gland, damaging tissues.
The work at UCL was supported by the European Research Council and the Royal Society. The work at Tufts was supported by the National Science Foundation and the US Department of Energy.
Notes
The study is published in a paper entitled "Enhancement of low-energy electron emission in 2-D radioactive films" published today in Nature Materials.
Related links
- Paper in Nature Materials
- 'News & views' article about the research in Nature Materials
- London Centre for Nanotechnology
- UCL Chemistry
- Thomas Young Centre
High resolution image
Artist's impression of an atom of iodine-125 decaying into an atom of tellurium-125. Credit: Angelos Michaelides and Philipp Pedevilla
Researcher profiles
Media contact
Oli Usher
UCL Faculty of Mathematical and Physical Sciences
020 7679 7964
o.usher@ucl.ac.uk
 Close
Close




