
Group members
Professor Michael Thomas - Co-Principal Investigator
Professor Denis Mareschal - Co-Principal Investigator
Dr Hana D'Souza - Postdoctoral Research Fellow
Dr Dan Brady - Postdoctoral Research Fellow
Kate Hughes - PhD student
Isabel Quiroz - Research Assistant
_________________
Formerly led by Professor Annette Karmiloff-Smith

Neurocognitive phenotypes of infants with Down syndrome: potential to predict protective/risk markers for Alzheimer's disease.
Although many individuals with Down syndrome (DS) do not
go on to develop the dementia associated with Alzheimer's disease (AD),
virtually all individuals with DS will develop the neuropathological features, such as amyloid plaques, common in
AD by the age of 30.
One proposed explanation is that the APP gene on
chromosome 21 plays a role, as it is over-expressed from infancy onwards.
However, other genes are also involved and it is still not fully understood why some individuals with DS develop AD dementia whilst others do not.
We argue that to understand any observable
characteristic, it is crucial to trace its features back to its origins in
early development.
Aims
The aims of the
current project are to understand individual differences during infancy that may
be associated with specific neurocognitive characteristics of AD.
A specific
focus will be on individual performances that are meaningfully above or below
the group average.
These individual differences may help to elucidate the protective and risk markers for AD.
Publicity
The project received a great deal of publicity when it was first announced in 2012 (take a look at the article in The Observer).
We have continued to share our research ideas with the scientific community since then (see our poster presented at the Cognition in Down Syndrome Workshop, Washington DC, USA, April 2013).
What we are doing
Nearly one-hundred and fifty infants between the
ages of 6 and 60 months have been recruited from Down syndrome organisations and
parental support groups.
A sub-group of these individuals will be followed
longitudinally to allow us to track specific neurocognitive phenotypes over
this developmentally-intense period.
We used non-verbal tasks including high-density electroencephalography (EEG see Fig. 1), to compute Event Related Brain Potentials (ERPs), and use eye-tracking techniques (see Fig. 2).
Figure 1
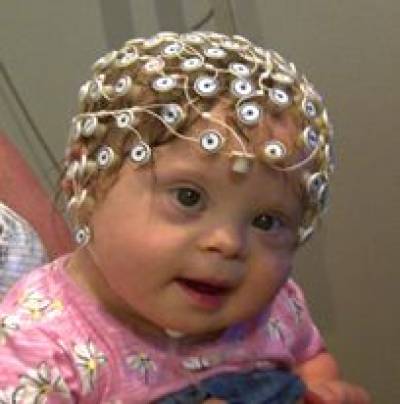
Infants wearing the EEG cap, a lightweight sensor cap that measures the electrophysiological activity coming out of the child's brain (like how a thermometer would measure temperature); nothing goes into the brain.
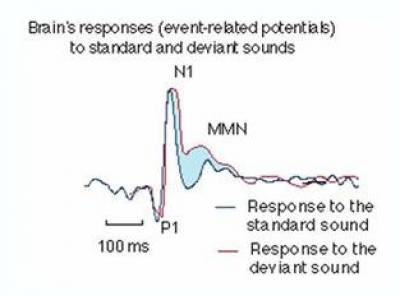
An average brain Event Related Potential collected from a group of infants' responses to repeated standard sounds versus unexpected deviant sounds (infants show memory discrimination for auditory stimuli).
Figure 2
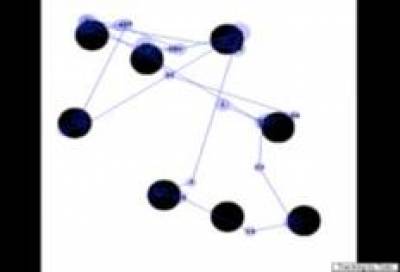
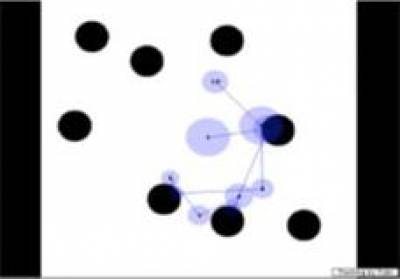
Results
from an eye-tracking experiment. The eye tracker is able to measure exactly
where the child is looking on the screen as the child scans a display.
Left:
Eye tracking data from an infant with Down syndrome;
Right:
infant with William syndrome.
We have also been measuring sleep patterns across the sleep/wake cycle using actigraphy, as we know that sleep plays a critical role in memory and neurocognitive development and that changes in sleep patterns precede AD symptoms in typically developing adults (see Fig 3).
Figure 3


Actigraphy watches are worn by infants on their ankle. They record movement and are used to monitor sleep/wake cycles.
These measures will be correlated with
questionnaires to investigate cognitive and socio-economic profiles with
respect to brain changes during infancy.
We envisage that our work could be of great benefit
to individuals with DS, by informing targeted, very early intervention strategies.
To learn more about our work or to ask any questions you may have, please email: downsyndrome@bbk.ac.uk
 Close
Close

