In interventional trials participants are assigned to receive one or more interventions (or no intervention) so researchers can evaluate the effects on biomedical or health-related outcomes.
Our interventional clinical trials happen in two ways: new research discoveries made in the Institute are tested in patients; or new drugs/ treatments developed by industry are tested in patients coming to the Institute.
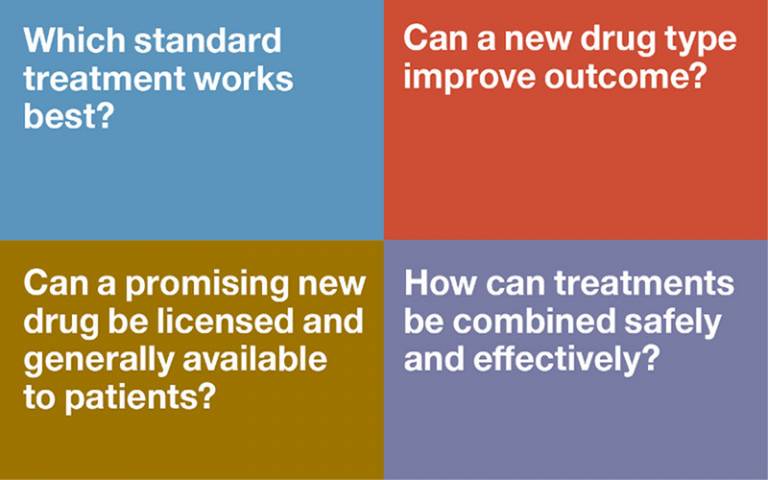
Studies that test the effectiveness of new or emerging treatments are vital to clinical prgoress. The Institute offers a robust and safe environment for cutting edge clinical trials.

Research discoveries in the Institute or other laboratories may suggest new potential drug treatments. These could ultimately lead to new licensed drugs, but the journey towards this is inevitably a long one. Initial stages focus on defining clinical potential and rationale.
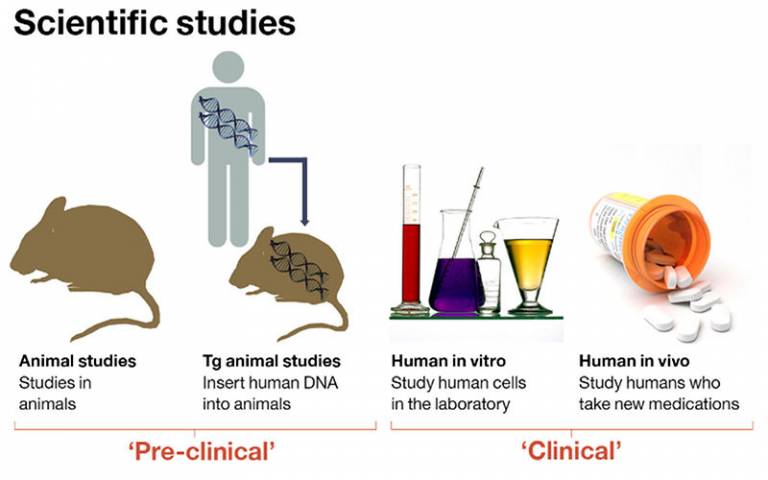
Studies of patient samples and disease models allow logical identification of new drug targets which can be test in clinical trials. Conversely, clinical trials provide an opportunity to better understand disease mechanism and suggest yet more potential new targets.
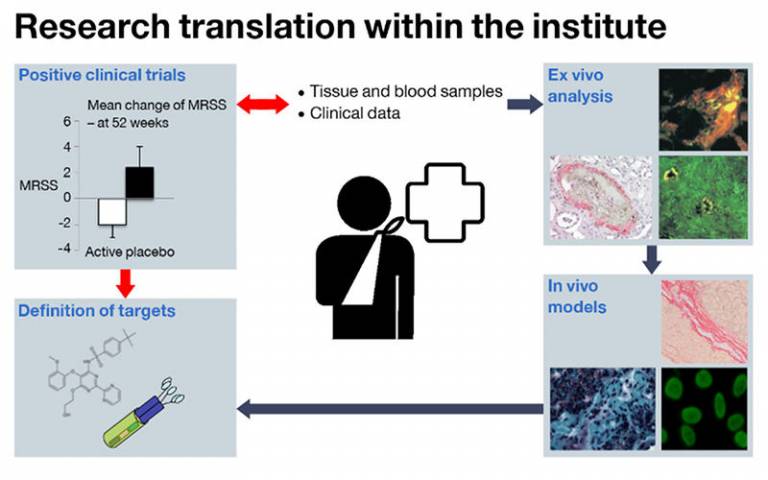
nce treatments have been shown to be effective, powerful opportunities to further define key aspects of disease biology are offered that may lead to more therapies.
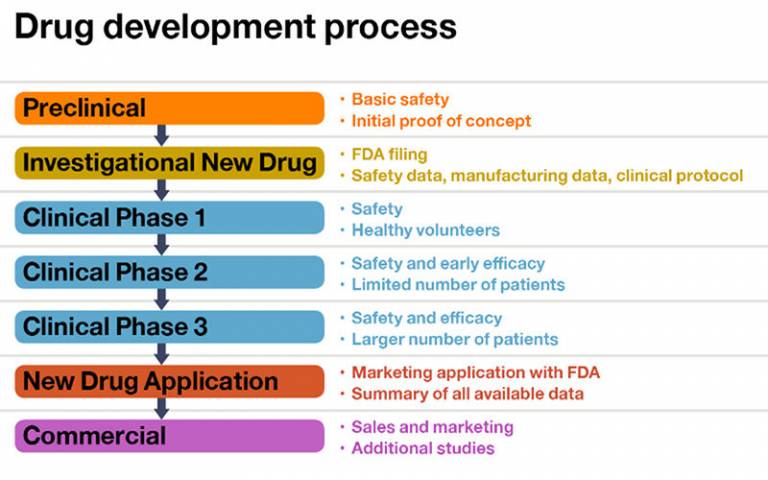
The drug development process starts with laboratory studies. Once a drug with a relevant mechanism has been discovered, it is usually tested for effect in animal models of the human disease, and subsequently in healthy human volunteers before progressing through dose and then effect studies in patients with the condition.

AIMSPRO® produced to GMP standards from serum obtained from a herd of certified “Scrapie-free” goats raised and housed at a TGA-approved facility in Tasmania. Animals are vaccinated using an inactivated HIV IIIB viral lysate / Serum is fractionated and nanofiltered / IMP is stored frozen for thawing and subcutaneous injection / Manufactured in an approved and MHRA audited facility in the UK.
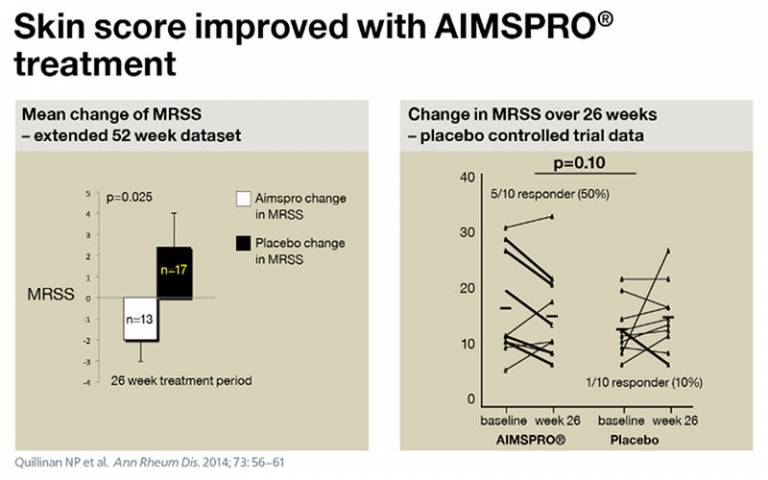
One recent trial performed in the Institute demonstrated that hyperimmune goat serum may improve skin fibrosis in established systemic sclerosis. Half of the patients treated showed improvement, compared to only one in ten receiving placebo.
 Close
Close

