- Introduction and nosology
- Synonyms
- Epidemiology
- Genetics
- Pathology
- Other focal posterior cortical syndromes
- Neuroimaging
- Management
- Conclusions
- References
Adapted from: Rohrer, Crutch, Warren and Rossor (in press).
Primary progressive aphasia and posterior cortical atrophy. In D Ames, A
Burns, J O'Brien (Eds), Dementia (4th Ed), Chapter 72.
Introduction and nosology
The term posterior cortical atrophy (PCA) refers to a progressive neurodegenerative condition involving prominent tissue loss in the posterior regions of the brain. Most commonly individuals with PCA exhibit a progressive, dramatic and relatively selective decline in higher visual processing and other posterior cortical functions. PCA is a descriptive term, introduced in the absence of pathological data identifying the cause of the earliest cases of the syndrome (Benson et al., 1988; Cogan et al., 1985; De Renzi, 1986). Subsequent histopathological studies have identified amyloid plaques and neurofibrillary tangles as the most common underlying pathology, leading to PCA being considered frequently as a variant of Alzheimer’s disease (AD). However, the occurrence of PCA associated with alternative etiologies has led to renewed calls for PCA to be considered as a distinct nosological entity with its own diagnostic criteria (Tang-Wai and Mapstone, 2006). Others suggest that, at least amongst patients with underlying Alzheimer’s disease, focal presentations such as PCA should be considered extremes of a continuum of variation in AD (Stopford et al., 2007).
Many questions remain over whether PCA should be considered a unitary clinico-anatomical syndrome or as a collection of related but distinct syndromic subtypes. Extrapolating from basic neuroscience evidence of distinct cortical streams which process different kinds of visual information (Ungerleider and Mishkin, 1982; Goodale and Milner, 1992), it has been suggested that separate parietal (dorsal), occipitotemporal (ventral) and primary visual (posterior) forms of PCA exist (Galton et al., 2000). One difficulty is that such claims are based upon the observation of patterns of impairment in single cases, and the existence of an occipitotemporal variant has not been supported by larger group studies (McMonagle et al., 2006). These anterior-posterior and superior-inferior distinctions also fail to capture the pronounced asymmetry apparent in the neuropsychological and neuroimaging profiles of many individuals with PCA (e.g. Freedman et al., 1991; Snowden et al., 2007). At a more general level, it is noteworthy that with a relative preservation of episodic memory, individuals with PCA do not meet all established criteria for dementia (e.g. DSM-IV).
Synonyms
Posterior cortical atrophy; Benson’s syndrome; progressive posterior
cortical dysfunction; biparietal Alzheimer’s disease; visual variant of
Alzheimer’s disease
Epidemiology
The exact prevalence and incidence of PCA are not known and any figure
is likely to be an underestimate because of poor general knowledge of
the syndrome’s existence. However, in a study of 523 patients with
Alzheimer’s disease at a single specialist centre, a visual presentation
(also labelled posterior cortical atrophy) was reported in 5% of the
cohort (Snowden et al., 2007). Studies comparing PCA and amnestic AD
suggest few epidemiological differences apart from age of disease onset,
which tends to be earlier in PCA around the mid 50s and early 60s (e.g.
Mendez et al., 2002; McMonagle et al., 2006) although some studies
report a wide distribution (40-86 years; Tang-Wai et al., 2004). Group
studies and reviews have indicated either no difference in prevalence
among the genders (e.g. Mendez et al., 2002, Renner et al., 2004;
McMonagle et al., 2006) or over-representation among women (e.g.
Tang-Wai et al., 2004; Snowden et al., 2007; Lehmann et al., 2009).
Genetics
The proportion of individuals with PCA with a positive family history of
dementia is not significantly different to individuals with typical AD
(Mendez et al., 2002; Tang-Wai et al., 2004). It is of note that there
have been no reports of an autosomal dominant inheritance pattern in
PCA, and indeed of the 11 PCA patients (27.5%) in the Tang-Wai et al.
(2004) study who had a family history of a dementing illness, none of
those family members had a posterior cortical syndrome. These studies
also report no difference in the ApoE status of PCA relative to typical
AD. However, a difference between the ApoE status of individuals with
posterior cortical presentations of AD and amnestic AD has been
suggested (Schott et al., 2006; Van der Flier, 2006; Snowden et al.,
2007). Schott et al. (2006) reported that fewer patients with biparietal
AD than typical AD have one or more ε4 alleles (20% and 86%,
respectively). In a larger study examining the relationship between
cognitive profile and ApoE status in 302 patients with typical or
atypical AD (Snowden et al., 2007), the percentage of patients with a
visual presentation possessing at least one ε4 allele was significantly
lower than patients with an amnestic presentation (30% and 82%
respectively; typical AD: 55%) and no different to a population of 756
healthy individuals from the same region (27%; Payton et al., 2003).
This evidence suggests that risk factors other than ApoE underpin
posterior cortical syndromes in AD.
Pathology
Histopathological studies have revealed that Alzheimer’s disease is the most common underlying cause of PCA (Hof et al., 1989, 1990; Ross et al., 1996; Galton et al., 2000; Tang-Wai et al., 2004; Renner et al., 2004; Snowden et al., 2007; Alladi et al., 2007). The distinction between PCA and typical AD lies in the distribution of this pathology. Compared to individuals with typical AD, patients with PCA show a much greater density of senile plaques and neurofibrillary tangles in occipital cortex and regions of posterior parietal cortex and temporo-occipital junction, whilst showing fewer pathological changes in more anterior areas such as prefrontal cortex (e.g. Levine et al., 1993; Ross et al., 1996; Hof et al., 1997). However, AD is not the only etiology responsible for the syndrome, with a small number of cases attributable to corticobasal degeneration (Tang-Wai et al., 2003a; Renner et al., 2004), Dementia with Lewy Bodies (Tang-Wai et al., 2003b; Renner et al., 2004), prion disease (including Creutzfeld-Jakob disease and familial fatal insomnia; Renner et al., 2004; Victoroff et al., 1994), and so-called subcortical gliosis (Victoroff et al., 1994).
Clinical and Neuropsychological features
Proposed diagnostic features
Two broadly comparable sets of diagnostic criteria have been proposed
(Mendez et al., 2002; Tang-Wai et al., 2004). Suggested core features
include: (i) insidious onset and gradual progression; (ii) presentation
with visual complaints in the absence of ocular disease; (iii)
relatively preserved episodic memory, verbal fluency and personal
insight; (iv) presence of symptoms including visual agnosia,
simultanagnosia, optic ataxia, ocular apraxia, dyspraxia and
environmental disorientation; (v) absence of stroke or tumour.
Supportive features include alexia, ideomotor apraxia, agraphia,
acalculia, onset before the age of 65 years and neuroimaging evidence of
posterior cortical atrophy or hypoperfusion.
Neurological signs
Neurological signs have been inconsistently reported in group studies of
PCA, and estimates of symptom frequency are dependent upon the
composition of the study population. However, among 24 PCA patients with
probable AD, the frequency of extrapyramidal signs (41%), myoclonus
(24%) and grasp reflex (26%) was found to be comparable to individuals
with typical AD (Snowden et al., 2007). Up to 25% of PCA patients may
also experience visual hallucinations (Tang-Wai et al., 2004; McMonagle
et al., 2006). In a series of 59 patients with PCA, visual
hallucinations were observed in 13 individuals (22%) and were associated
with parkinsonism, rapid eye movement sleep behaviour disorder,
myoclonic jerks and not only atrophy of occipito-parietal regions but
also disruption of thalamocortical circuits (Josephs et al., 2006).
Neuropsychological profile
The most frequently cited neuropsychological deficits in PCA are
visuospatial and visuoperceptual deficits, including some or all of the
features of Balint’s syndrome (simultanagnosia, oculomotor apraxia,
optic ataxia, environmental agnosia), Gerstmann’s syndrome (acalculia,
agraphia, finger agnosia, left/right disorientation), alexia, agraphia,
acalculia and apraxia (Mendez et al., 2002; Tang-Wai et al., 2004;
Renner et al., 2004; Charles and Hillis, 2005; McMonagle et al., 2006;
Whitwell et al., 2007). The most detailed neuropsychological study of
PCA to date suggests that of these symptoms, alexia, agraphia,
simultanagnosia and optic ataxia are the most consistently identified
features. Additional features reported in a proportion of patients
include aganosia for objects, faces and colours, but overall the pattern
of impairments is suggestive of greater impairment of the dorsal than
ventral visual processing streams as no pure ventral stream syndromes
were detected (McMonagle et al., 2006). However, a clear distinction
exists between patients who show predominantly parietal deficits and
those who show early impairments of early visual processing skills (e.g.
figure-ground discrimination, shape discrimination, visual crowding;
e.g. Crutch and Warrington, 2007) associated with atrophy of the striate
and extrastriate cortex (e.g. Galton et al., 2000). Overall, the
plethora of associated posterior cognitive deficits have predictable
consequences for the performance of PCA patients on more general
neuropsychological tests such as performance IQ (often up to 30-40
points lower than verbal IQ scores) and constructional tasks (e.g. Rey
figure copy; clock drawing). Longitudinal studies have demonstrated that
anterograde memory functions, linguistic skills and frontal lobe
functions, which are sometimes strikingly preserved in the earlier
stages of the condition, do gradually deteriorate as individuals
progress to a more global dementia state (e.g. McMonagle et al., 2006;
Levine et al., 1993). The aphasic difficulties are characterised by
progressive anomia and phonological impairment and increasingly resemble
the LPA syndrome described above.
Unusual symptoms
Whilst individuals with PCA experience the loss of many visual
functions, many also describe unusual new experiences, or ‘positive
perceptual phenomena’. These phenomena have not been widely recognised,
but include abnormally prolonged colour afterimages (Chan et al., 2001),
reverse size phenomena (e.g. Stark et al., 1997), the perception of
movement among static stimuli, and in one case even the 180˚ upside-down
reversal of vision (Crutch et al., submitted). Anecdotally, individuals
with PCA also report a range of localised sensory and pain phenomena,
and disturbances of balance and bodily orientation potentially linked to
deranged visuo-vestibular interactions. Detailed investigation of these
positive symptoms could potentially yield important new insights about
the pathophysiology as well as the phenomenology of PCA.
Other focal posterior cortical syndromes
The proposed diagnostic criteria for PCA (Mendez et al., 2002; Tang-Wai
et al., 2004) both consider ‘presentation with visual complaints’ as a
core feature of the syndrome. However, some patients with Alzheimer’s
disease present initially with focal deterioration in other cognitive
domains such as praxis or spelling (e.g. De Renzi, 1986; Green et al.,
1995; Aharon-Peretz et al., 1999; Snowden et al., 2007) with relative
early sparing of visual function. Often these individuals then progress
to a more general posterior cortical syndrome with memory relatively
spared until later in the disease course, and consequently could be
considered to fall within the spectrum of PCA phenotypes. Given the
increasing awareness of non-AD pathologies underpinning PCA and the move
toward considering PCA as a distinct nosologic entity in its own right,
it may be that the available diagnostic criteria for PCA will require
some expansion to include posterior cortical presentations which are not
primarily visual in nature.
Neuroimaging
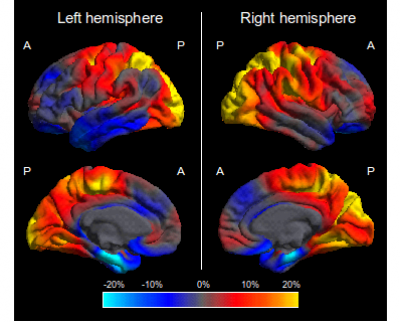
As the term PCA suggests, the syndrome is associated with tissue loss primarily of the occipital, parietal and temporo-occipital cortices. This pattern of atrophy is often evident from visual inspection of structural MR or CT images, but less specific radiological findings such as generalised atrophy or normal volume for age are also common (e.g. Della Sala et al., 1996; Galton et al., 2000; Mendez et al., 2002). To date, only two systematic evaluations of brain structure in PCA using automated methods have been conducted, with voxel-based morphometry revealing greater right parietal and less left medial temporal and hippocampal atrophy in PCA patients compared with those with typical AD (Whitwell et al., 2007; Lehmann et al., 2009). In addition, direct cortical thickness comparisons between PCA and typical AD revealed greater cortical thinning in the right superior parietal lobe and less thinning in the left entorhinal cortex among PCA patients (Lehmann et al., 2009; see Figure 1).
Consistent with these structural findings of atrophic changes, functional imaging techniques such as SPECT and PET imaging often reveal hypoperfusion and indicate hypometabolism in dorsal occipito-parietal more than ventral occipito-temporal regions (e.g. Pietrini et al., 1996; Aharon-Peretz et al., 1999; Goethals and Santens, 2001). In addition to posterior regions, FDG-PET has revealed specific areas of hypometabolism in the frontal eye fields bilaterally which may occur secondary to loss of input from occipito-parietal regions and underpin ocular apraxia in PCA (Nestor et al., 2003). Pathophysiological studies using fMRI are lacking in PCA, and may be especially pertinent in PCA as the positive perceptual phenomena described by these patients suggest not merely loss of activity but aberrant activity in affected cortical areas. Preliminary applications of novel imaging methods such as diffusion tensor imaging (DTI; Yoshida et al., 2004; Duning et al., 2009) and amyloid imaging (Tenovuo et al., 2008) have been reported in single cases, showing reductions in fractional anisotropy (FA) and increased amyloid-beta accumulation in the occipital and parietal lobes.
You can read more about neuroimaging in PCA on the brain imaging page of this site.
Management
There are currently no published studies examining the effectiveness of acetyl cholinesterase therapy specifically in PCA. However, anecdotal and limited single case reports suggest that donepezil, rivastigmine and galantamine are associated in symptomatic benefit in a proportion of the population (e.g. Kim et al., 2005), most likely those individuals with an underlying pathological diagnosis of Alzheimer’s disease or Dementia with Lewy Bodies.
A lack of understanding of PCA in the wider community limits patients’
access to relevant services, and the care and advice which is provided
is often inappropriate, catering to problems that are not significant
(e.g. memory deficits) whilst failing to cater to functionally critical
perceptual deficits (e.g. many activities in day centres and nursing
homes are visually mediated). Owing to the relative preservation of
memory, language and personal insight particularly in the mild and
moderate stages of the condition, individuals with PCA are well-disposed
to take advantage of peer support meetings and group, couple and
individual psychological therapies where the need exists. Peer support
meetings in particular provide an important opportunity for reducing
social isolation, sharing the experience of what is often a particularly
long and difficult route to diagnosis, and for exchanging practical
tips and advice for managing problems associated with the condition.
Individuals with PCA often benefit from resources designed primarily for
the blind and partially sighted, such as talking watches, mobile phones
with simplified displays, voice recognition software, talking books,
culinary aids, and lamps to increase ambient light levels in the home.
Referral to an ophthalmologist may also be required for an individual to
register as partially sighted under statutory invalidity schemes, which
can enable access to financial and social benefits and services.
Conclusions
Posterior cortical atrophy is a debilitating and under-recognised focal degenerative condition which is associated with a range of different disease pathologies. PCA at once provides a crucial window on the neurobiology of neurodegeneration and raises substantial nosological difficulties. The PCA syndrome justifies an independent nosological status, but with Alzheimer’s disease as the most common underlying cause, a lack of consistency between studies regarding the classification of PCA at the disease or syndrome level is likely to continue until more detailed diagnostic criteria and terminology are available. Better understanding and awareness of the syndrome among the medical and lay communities is necessary to improve the support services and information provided to individuals with PCA and their families. Dedicated clinical trials assessing the effectiveness of pharmacological and non-pharmacological interventions are also required in this small but significant population of patients with degenerative conditions.
Manja Lehmann gave a presentation about research being carried out to
investigate Posterior Cortical Atrophy at a support group meeting
(November 2010). To read the research presentation you need a PDF reader, you can download Adobe Reader here for free.
References
- Aharon-Peretz J, Israel O, Goldsher D, Peretz A. Posterior cortical atrophy variants of Alzheimer's disease. Dementia and Geriatric Cognitive Disorders. 1999;10(6):483-487.
- Alladi S, Xuereb J, Bak T, Nestor P, Knibb J, Patterson K, Hodges JR. Focal cortical presentations of Alzheimer's disease. Brain. 2007 Oct;130(Pt 10):2636-45.
- Benson F, Davis J, Snyder BD. Posterior Cortical atrophy. Arch Neurol. 1988;45:789-793.
- Chan D, Crutch SJ, Warrington EK. A disorder of colour perception associated with abnormal colour after-images: a defect of the primary visual cortex. Journal of Neurology Neurosurgery and Psychiatry. 2001;71(4):515-517.
- Charles RF, Hillis AE. Posterior cortical atrophy: clinical presentation and cognitive deficits compared to Alzheimer's disease. Behav Neurol. 2005;16(1):15-23.
- Cogan DG. Visual Disturbances with Focal Progressive Dementing Disease. American Journal of Ophthalmology. 1985;100(1):68-72.
- Crutch SJ, Warrington EK. Foveal crowding in posterior cortical atrophy: a specific early-visual-processing deficit affecting word reading. Cogn Neuropsychol. 2007;24(8):843-866.
- Crutch SJ, Lehmann M, Gorgoraptis N, Kaski D, Ryan N, Husain M & Warrington EK. Abnormal visual phenomena in posterior cortical atrophy. Submitted.
- Della Sala S, Spinnler H, Trivelli C. Slowly progressive impairment of spatial exploration and visual perception. Neurocase. 1996;2(4):299-323.
- De Renzi E. Slowly progressive visual agnosia or apraxia without dementia. Cortex. 1986;22(1):171-180.
- Duning T, Warnecke T, Mohammadi S et al. Pattern and progression of white-matter changes in a case of posterior cortical atrophy using diffusion tensor imaging. J Neurol Neurosurg Psychiatry. 2009;80(4):432-436.
- Freedman L, Selchen DH, Black SE et al. Posterior cortical dementia with alexia: neurobehavioural, MRI, and PET findings. J Neurol Neurosurg Psychiatry. 1991;54(5):443-448.
- Galton CJ, Patterson K, Xuereb JH, Hodges JR. Atypical and typical presentations of Alzheimer's disease: a clinical, neuropsychological, neuroimaging and pathological study of 13 cases. Brain. 2000 Mar;123 Pt 3:484-98.
- Goethals M, Santens P. Posterior cortical atrophy. Two case reports and a review of the literature. Clinical Neurology and Neurosurgery. 2001;103(2):115-119.
- Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends Neurosci. 1992;15(1):20-25.
- Green RC, Goldstein FC, Mirra SS et al. Slowly Progressive Apraxia in Alzheimers-Disease. Journal of Neurology Neurosurgery and Psychiatry. 1995;59(3):312-315.
- Hof PR, Bouras C, Constantinidis J, Morrison JH. Balint's syndrome in Alzheimer's disease: specific disruption of the occipito-parietal visual pathway. Brain Res. 1989;493(2):368-375.
- Hof PR, Bouras C, Constantinidis J, Morrison JH. Selective disconnection of specific visual association pathways in cases of Alzheimer's disease presenting with Balint's syndrome. J Neuropathol Exp Neurol. 1990;49(2):168-184.
- Hof PR, Vogt BA, Bouras C, Morrison JH. Atypical form of Alzheimer's disease with prominent posterior cortical atrophy: a review of lesion distribution and circuit disconnection in cortical visual pathways. Vision Res. 1997;37(24):3609-3625.
- Josephs KA, Whitwell JL, Boeve BF et al. Visual hallucinations in posterior cortical atrophy. Arch Neurol. 2006;63(10):1427-1432.
- Lehmann M, Crutch SJ, Ridgway GR et al. Cortical thickness and voxel-based morphometry in posterior cortical atrophy and typical Alzheimer's disease. Neurobiology of Aging. 2009. doi:10.1016/j.neurobiolaging.2009.08.017.
- Levine DN, Lee JM, Fisher CM. The visual variant of Alzheimer's disease: a clinicopathologic case study. Neurology. 1993;43(2):305-313.
- McMonagle P, Deering F, Berliner Y, Kertesz A. The cognitive profile of posterior cortical atrophy. Neurology. 2006;66(3):331-338.
- Mendez MF, Ghajarania M, Perryman KM. Posterior cortical atrophy: Clinical characteristics and differences compared to Alzheimer's disease. Dementia and Geriatric Cognitive Disorders. 2002;14(1):33-40.
- Nestor PJ, Caine D, Fryer TD et al. The topography of metabolic deficits in posterior cortical atrophy (the visual variant of Alzheimer's disease) with FDG-PET. J Neurol Neurosurg Psychiatry. 2003;74(11):1521-1529.
- Pietrini P, Furey ML, GraffRadford N et al. Preferential metabolic involvement of visual cortical areas in a subtype of Alzheimer's disease: Clinical implications. American Journal of Psychiatry. 1996;153(10):1261-1268.
- Renner JA, Burns JM, Hou CE et al. Progressive posterior cortical dysfunction: a clinicopathologic series. Neurology. 2004;63(7):1175-1180.
- Ross SJ, Graham N, Stuart Green L et al. Progressive biparietal atrophy: an atypical presentation of Alzheimer's disease. J Neurol Neurosurg Psychiatry. 1996;61(4):388-395.
- Schott JM, Ridha BH, Crutch SJ et al. Apolipoprotein E genotype modifies the phenotype of Alzheimer disease. Arch Neurol. 2006;63(1):155-156.
- Snowden JS, Stopford CL, Julien CL et al. Cognitive phenotypes in Alzheimer's disease and genetic risk. Cortex. 2007;43(7):835-845.
- Stark ME, Grafman J, Fertig E. A restricted 'spotlight' of attention in visual object recognition. Neuropsychologia. 1997;35(9):1233-1249.
- Stopford CL, Snowden JS, Thompson JC, Neary D. Distinct memory profiles in Alzheimer's disease. Cortex. 2007;43(7):846-857.
- Tang-Wai D, Mapstone M. What are we seeing? Is posterior cortical atrophy just Alzheimer disease? Neurology. 2006;66(3):300-301.
- Tang-Wai DF, Josephs KA, Boeve BF et al. Coexistent Lewy body disease in a case of "visual variant of Alzheimer's disease". J Neurol Neurosurg Psychiatry. 2003;74(3):389.
- Tang-Wai DF, Josephs KA, Boeve BF et al. Pathologically confirmed corticobasal degeneration presenting with visuospatial dysfunction. Neurology. 2003;61(8):1134-1135.
- Tang-Wai DF, Graff-Radford NR, Boeve BF et al. Clinical, genetic, and neuropathologic characteristics of posterior cortical atrophy. Neurology. 2004;63(7):1168-1174.
- Tenovuo O, Kemppainen N, Aalto S et al. Posterior Cortical Atrophy: A Rare Form of Dementia with in vivo Evidence of Amyloid-beta Accumulation. Journal of Alzheimers Disease. 2008;15(3):351-355.
- Ungerleider LG, Mishkin M. Two cortical visual systems. In: Ingle DJ, Mansfield RJW, Goodale MD, editors. The analysis of visual behaviour. Cambridge, MA: MIT Press, 1982: 549-86.
- Victoroff J, Ross GW, Benson DF et al. Posterior Cortical Atrophy - Neuropathologic Correlations. Arch Neurol. 1994;51(3):269-274.
- Whitwell JL, Jack CR, Kantarci K et al. Imaging correlates of posterior cortical atrophy. Neurobiology of Aging. 2007;28(7):1051-1061.
- Yoshida T, Shiga K, Yoshikawa K et al. White matter loss in the splenium of the corpus callosum in a case of posterior cortical atrophy: A diffusion tensor imaging study. European Neurology. 2004;52(2):77-81.
 Close
Close

