Red Cabbage pH-indicator
Overview
Make your own pH-indicator, using red cabbage.
Equipment
- 1/2 head of red cabbage
- a metal grater
- cooking pot, filled with some water
- strainer
- glass or jar
- some acids and/or bases: lemon, vinegar, detergent...
Safety
- Be careful when grating the red cabbage
Procedure of experiment
- Grate the red cabbage into small pieces and place them into the pot. Add water until the cabbage is covered with it.
- Boil the cabbage for 20 to 30 minutes until the liquid has a dark purplish colour.
- Decant the fluid into a glass or a jar through a strainer to remove the cabbage. Do not throw away the cabbage; you can still eat it.
- Make some test solutions which are either acidic or basic. Also have a "test" solution; water should be fine (although water is slightly acidic.)
- Add a few drops of the cabbage juice to your solutions and write down the colour changes. You now have your own pH-indicator!
Explanation
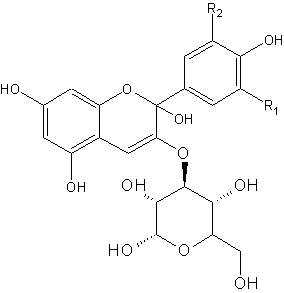
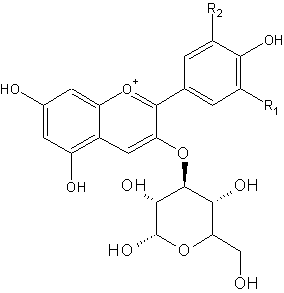 Red cabbage contains pigments called anthocyanins. These pigments give the purplish colour. On the right side is the general chemical structure of anthocyanins. R1 and R2 can be different chains, like H, OH and OMe.
Red cabbage contains pigments called anthocyanins. These pigments give the purplish colour. On the right side is the general chemical structure of anthocyanins. R1 and R2 can be different chains, like H, OH and OMe.
| R1 | R2 |
| cyanidin | H | OH |
| delphinidin | OH | OH |
| malvidin | O-CH3 | O-CH3 |
| pelargonidin | H | H |
| peonidin | H | O-CH3 |
| Petunidin | OH | O-CH3 |
The anthocyanin present in red cabbage is cyaniclin. Some food sources containing anthocyanins are shown below. In an acidic environment, the left form is the more abundant, whereas in a basic environment, more of the right form is present. Both molecules have a different colour, hence we see a change in colour.
| apple | cyanidin |
| blueberry | malvidin |
| petuniclin |
| delphinidin |
| cranberry | cyanidin |
| peonidin |
| red cabbage | cyaniclin |
| strawberry | pelargonidin |
| cyanidin |
For most pH indicators, the compound acquires a proton at low pH (lots of H+) but looses it at higher pH. This seemingly minor alteration is sufficient to alter the wavelengths of light reflected by the compound, thus creating the color change with respect to pH. Anthocyanins behave somewhat inversely in that the pigments "gain" an -OH at basic pH, but loose it at acidic pH.
An acidic solution contains an excess of protons or H+. pH is a measure of how 'acidic' a solution is. The lower the pH, the more acidic the solution. In chemical terms, pH means "the negative log of the concentration of protons" in solution. Chemistry students should recognize this as
pH = -log[H+]. If the concentration of H+ is 0.01M, the pH will be:
-log[0.01] = -log[10-2] = -(-2) = 2 (which is very acidic!).
"Neutral" solutions (water, e.g.) have a pH of 7. This number coicides with the amount of H+ naturally formed in water from the equilibrium reaction: H2O <--> H+ + OH- (H+ experimentally known to be ~10-7M; OH- is also the same concentration). "Basic" solutions have a pH greater than 7 - meaning they have less free H+ than that of neutral water.
Previous Index Next

 Red cabbage contains pigments called anthocyanins. These pigments give the purplish colour. On the right side is the general chemical structure of anthocyanins. R1 and R2 can be different chains, like H, OH and OMe.
Red cabbage contains pigments called anthocyanins. These pigments give the purplish colour. On the right side is the general chemical structure of anthocyanins. R1 and R2 can be different chains, like H, OH and OMe.