Planning for Biosafety Environments - control of infectious disease in projects
We provide an online series of short courses, supported by UCL Life Learning, for all those involved in the planning and design of biosafety environments.
An interprofessional team of experts in the fields of microbiology, medicine, design, engineering and project requirements offer the latest research and knowledge in diagnostic, treatment and investigative environments where infectious diseases need to be contained and controlled.
- Overview
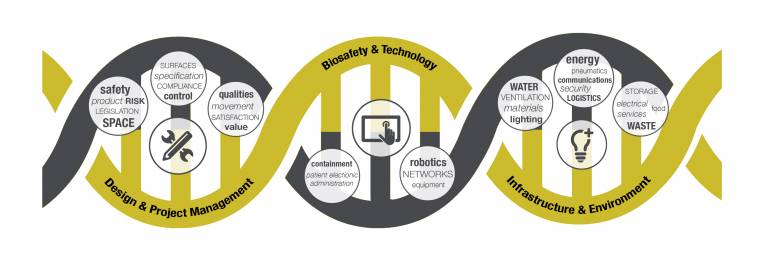
From designing, building and fitting a new laboratory, to setting up ultra-clean environments such as surgical theatres or acute and emergency care in hospitals: the processes and knowledge needed at all stages of a project are complicated, varied and constantly evolving with the latest technology and research.
One of the greatest challenges in such projects is to understand the expectations, abilities and scope of the different stakeholders involved and to find a common 'language' in which to communicate.
A project could involve architects, construction companies, equipment manufacturers, medical staff, scientists and project managers. Each specialism has different levels of knowledge, understanding, abilities and vocabulary.
How can you ensure a project not only exploits the latest research and technology available, but also operates smoothly and has a successful outcome when so many different specialisms and requirements are involved?
Experts at UCL have come together in a unique cross-disciplinary approach to training all key stakeholders involved in infection control so that, regardless of your role or industry related to this subject, you can understand and use the latest approaches in a development project.
If you complete the full course, you'll learn how to apply the latest research and transformational thinking to the design and management of the environment, and will have the opportunity to develop on your own work-based project under the guidance of UCL experts and a multi-disciplinary peer-group.
A common theme throughout the course will be the skills needed to communicate with all parties involved and how to find a common language.
The course is designed and delivered by leading academics in below groups at UCL. They have extensive microbiological, clinical, design, project and construction management experience, combined with state of the art research and industry expertise in the field.
- UCL -TB
- UCL Centre for Clinical Microbiology
- The Bartlett School of Construction & Project Management
- UCL Civil, Environmental & Geomatic Engineering
We are also support by experts and our academic partners in the field of infection control from University College London NHS Hospitals, Great Ormond Street Hospital for Children and The Royal Free London NHS Trust.
- Course content
Four online modules of inter-disciplinary training with peer to peer interactive discussion groups on how to apply the latest evidence with transformational thinking on the design of biosafety environments, and to ensure the perspective of all the stakeholders are considered.
- Module 1 Principles of biosafety design,
- Module 2 Measurement of infectious diseases ,
- Module 3 Project evaluation for clean and controlled environments,
- Module 4 Designing diagnostic processes and space,
Case studies, and work-based scenarios will include biotechnical and pharmaceutical laboratories, haematology and aseptic units, ultra-clean theatres, inpatient wards and high security treatment units, diagnostic testing and screening across health systems from emergency departments to mobile clinics.- Additional option, Module 5 Interactive work-based project evaluation
Module 5 is an online/blended to develop your work-based projects under the guidance of the UCL team and course peer-group. The aim to ensure a project successfully exploits the latest research and technology available, but also operates smoothly when so many different project requirements are involved.
UCL will host the module, planned over a 2 day period in London and if there is a demand we will host a second session is proposed with our academic and scientific partners in Africa.
- Module outline
Module 1 Principles of Biosafety Design
- principles of biosafety design across biosafety environments
- infection control policies, regulations and guidance all specialisms
how to review biosafety in different healthcare settings and understand the basic principles of hygiene in transmission reduction, infection prevention and control for the following objectives:-
- Isolation and treatment of infectious and infected patients
- Protection of medical and nursing staff treating the patients and microbiology staff investigating the infections
- Prevention of cross-infection within a healthcare facility
- Risk to the general community to infectious disease outbreak
- design processes, project management organsiation for the design, engineering and construction of facilities for the control of infection.
- 'state of the art' design, technical standards and legislation.
Module 2 Measurement of infectious diseases
- bio containment classifications relative to the surrounding environment
- types of disease related to biosafety levels
- procedures for agents of potential hazard to personnel and the environment
- techniques in data collection
- handling pathogenic agents, including dangerous and exotic agents that pose a high individual risk in transmission.
- access to, and design of, laboratory environments where work is being conducted
- precautions and procedures in handling specialised instruments and equipment for infectious environments, including the use and specification of physical containment or biohazard equipment and services.
- isolation facilities
- what will inhibit an outbreak of pathogens e.g. ultra clean theatres, aseptic environments & pharmacies.
Module 3 Project evaluation for clean and controlled environments
- tools and techniques for evaluating microbiological containment
- risk analysis / procedures
- building design protocols and tools, evaluating the performance of an existing space
- how infection prevention influence the design and management of a facility (rather than be applied retrospectively to a design).
- policy and regulatory framework but also to understand design guidance that is open to interpretation: 'design 'dilemmas'
- project requirements, regulations and legislation relating to biotechnical building standards with containment
- case studies to illustrate principles and concepts will include, isolation facilities (in emergency departments), decontamination suites, clean preparation and dirty utilities, provision of equipment cleaning and waste disposal, hand washing facilities (including WHO regulations).
Module 4 Designing diagnostic processes and space
- early detection and prevention of pathogen transmission in a range of healthcare settings
- progressive testing and screening technologies
- diagnostic testing integrated with spatial and infrastructural designs
- mobile screening and systems
- transform diagnostic infrastructure
- develop protocols for new ways to use technological innovation in diagnostic and screening environments.
- creating a route map to automatic point of care testing
- review novel visualisation analytics from collected data.
- setting systems (re)design requirements with positive impacts on service users safety in a variety of care settings
Module 5 Interactive work-based project evaluation
2 day interactive blended content UCL/Africa: online and/or face-to-face workshop for those with, or intending to start, a work-based project in the field.
- submit project outline in advance of the Module in preparation for the expert support and peer review
- informing policy and design standards within healthcare organisations
- Course structure
How is the course structured, taught and assessed?
- A key element of the course is communicating within a multi-disciplinary environment. You therefore can't complete this course on your own, in your own time. You'll be part of a group and will need to log into the course at specific times for learning or discussion.
- If you're based in a time zone that is not compatible with the course timings, or miss a session, you'll be able to access recordings of sessions and tutors by arrangement, but may not be able to take part in the active, live discussions.
Module format - each Module 10 hours total
Lectures will be uploaded prior to the Module Commnnccing. Lecture sessions will be introduced with interactive discussion forum
Generic format, general start of sessions 14.00 GMT
Monday: Pre course reading preparation, Introduction, Regulation, Compliance and Innovation updates, Lectures with interactive discussion - 2 hours total
Tuesday: Lectures with interactive discussion and Work-based Case Studies - 2 hours + 2 hours Scenario based exercises
Wednesday: Online test - 1 hour
Thursday: Assessment, Results and Student Peer Reflection Forum, Individual Online tutorials - 2 hours
Content will be supported by the occasional webcasts and podcasts produced by the team.
- Who should attend?
This course is aimed at a broad spectrum of specialists involved in the design, construction and management of acute infection-control environments such as laboratories, clinical theatres and wards that are 'ultra-clean'.
We recommend you have some prior experience or knowledge of biosafety environments or projects.
In clinical and medical settings:
- Microbiologists
- Bio security & infectious disease and control managers
- Diagnostic and pathology teams
- Clinical and medical staff from multiple disciplines, including those working in a military or humanitarian relief context
- Technology & equipment commissioners
In management:
- Planners and project managers
- Healthcare and laboratory managers, including buildings/estate managers
- International development and funder teams, including financial, governmental, NGOs and charitable agencies
- Construction design managers
- Facilities managers
- Operational managers
In design, construction and manufacturing:
- Architects
- Construction planning
- Mechanical, environmental and electrical engineers and designers
- Infectious diseases transmission and simulation modellers
- Custodial care designers and managers
- Ultraclean environments and equipment designers
- Learning outcomes
Knowledge and understanding
- Develop understanding and link the principles, concepts and tasks of biosafety delivery and design through analysis and investigation of new research and technologies
- Develop knowledge of policies, regulations, standards and guidance from the perspectives and interface of all specialisms
Intellectual skills
- Appraise principles for project information requirements and data from a variety of sources and case study examples, and discern and establish connections
- Apply professional judgement and devise transformational decisions in understanding the basic principles of infectious disease prevention and control
Transferable skills
- Communicate principles of biosafety, complex ideas, information and data to peers by oral, written and visual means
- Course benefits
Benefits for students
If you attend this course you'll
- Have access to a unique multi-disciplinary approach to biosafety design in infectious disease prevention and control that encompasses microbiology, clinical, design and project management expertise.
- Learn from world leading experts in these fields.
- Interact with a multi-disciplinary peer group that can prepare you for working on projects within your normal working environment. The cohort-based learning means you'll be able to build relationships with your peers and develop a support network to help you when applying the learning into your work environment. You'll be able to build relationships and understanding with key stakeholders, clients and suppliers involved in these kinds of projects.
- Benefit from transformative thinking and gain a new perspective on your projects.
- Access the most up-to-date thinking, research and technology related to this area.
Benefits for employers
If you enable your employees to attend this course, your organisation will benefit by:
- Receiving the latest evidence base, legislation and guidance updates.
- Increasing the likelihood of project success due to the knowledge, experience and confidence your staff gain on this course.
- Reduced risk and costs involved in carrying out such projects due to efficiencies and better planning.
- Having access to a relevant, experience peer and expert network for support and knowledge-sharing.
Accreditation and certificates available
You'll receive a UCL Certificate of Participation for your Continuing Professional Development credits.
 Close
Close


