
Two groups of coenzymes react directly with substrates: the nicotinamide nucleotides and the flavin coenzymes. In addition, the electron transport chain contains ubiquinone, cytochromes and non-haem iron proteins. The nicotinamide nucleotides, flavins and ubiquinone all undergo oxidation / reduction reactions reactions involving transfer of both hydrogen ions and electrons; the cytochromes and non-haem iron proteins undergo oxidation / reduction reactions reactions involving transfer of electrons only.
In NAD (nicotinamide adenine dinucleotide) and NADP (nicotinamide adenine dinucleotide phosphate), the reactive group is the nicotinamide ring. In the oxidised coenzymes this has a positive charge; reduction is always a two-electron process, resulting in formation of NAD(P)H and an associated proton.

In different enzymes flavins may be present as covalently-bound riboflavin, or tightly (but non-covalently) bound riboflavin monophosphate (which is sometimes known as flavin mononucleotide, FMN) or flavin adenine dinucleotide (FAD).

The flavin coenzymes can undergo redox reactions either as a single two-electron reaction or as two single-electron reactions, by way of an intermediate flavin free radical.

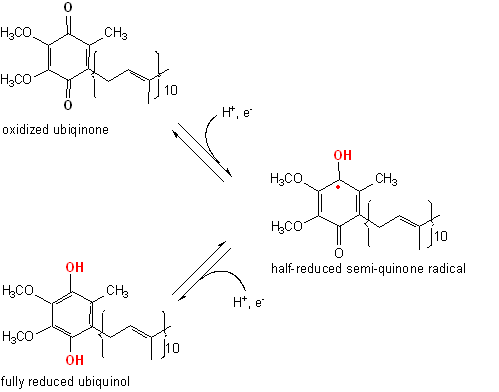
Ubiquinone undergoes a two-electron redox reaction in two single electron steps, via a half-reduced semiquinone radical.
The cytochromes consist of a tetrapyrrole (porphyrin) ring chelating a central iron atom; in the non-haem iron proteins the iron atoms are chelated by sulphydryl groups of cysteine residues and inorganic sulphide (hence the alternative name of iron sulphur proteins). In both cases the iron atom undergoes a single-electron redox reaction.
The haems in different cytochromes differ in the substituents in the porphyrin ring. This affects both their redox potential and also permits different methods of binding to the proteins in different cytochromes:
